Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Oxidation Reactions in Organic Chemistry
11.3 Oxidation of Alcohols and Aldehydes
Some of the oxidizing agents that are typically used in organic chemistry include H2CrO4/H2SO4, KMnO4/KOH, CrO3/pyridine, and CrO3/H2SO4. The last reagent, CrO3/H2SO4, is a very common oxidizing agent and is often referred to as the Jones reagent. Note that all these agents have oxygen and at least three oxygen atoms. An oxidizing agent, however, does not necessarily need to have oxygen atoms, but most that are used in organic chemistry have oxygen atoms, and hence is a very good indication that a particular agent is an oxidizing agent. In this section, we will examine the oxidation of molecules that have specific functional groups, alcohols and aldehydes. The first set of molecules that will be examined are alcohols. Alcohols that have at least one hydrogen atom bonded to the carbon of the alcohol functionality (−OH) can be oxidized to different functionalities, which include aldehydes, ketones, and even carboxylic acids. That is, methanol, primary and secondary alcohols can be oxidized but not a tertiary alcohol since tertiary alcohols have three alkyl groups bonding to the carbon of the alcohol functionality and no hydrogen. Aldehydes that are the products of an alcohol oxidation can be oxidized further to give another molecule with a different functionality, carboxylic acids. Aldehydes have a hydrogen atom bonded to the carbonyl carbon functionality, which makes it possible to be oxidized further.
Consider the oxidation reaction shown in Reaction (11-10), a close examination shows that a hydrogen atom is lost in going from the reactant (alcohol) to the organic product (ketone). Note that it is not just any hydrogen from the molecule that is lost, but the hydrogen that is bonded to the carbon of the alcohol functionality, along with the hydrogen from the alcohol.
(11-10)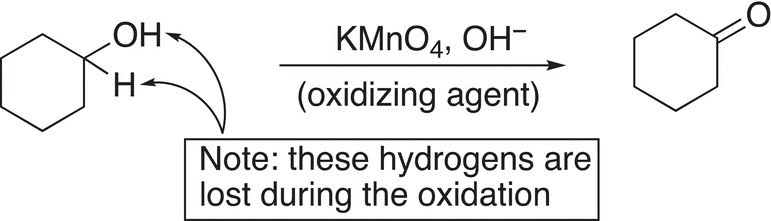
In the next section, we will examine the mechanism for oxidation reactions and account for the loss of the hydrogens and change of oxidation state of the carbon of the functional group being oxidized.
11.3.1 Oxidation Using Potassium Permanganate (KMnO4)
An examination of the reaction mechanism will show how the loss of the hydrogen atoms occurs during the oxidation. During the first step of the mechanism, the nucleophilic oxygen of the alcohol functionality adds to the very electrophilic manganese of KMnO4 followed by the loss of water as shown in Reaction (11-11).
(11-11)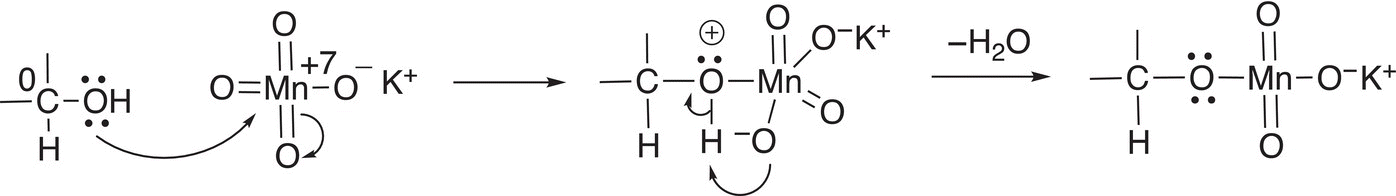
In the next step of the mechanism, the hydrogen that is bonded to the alcohol carbon is abstracted by the base to release the oxidized carbonyl compound as shown in Reaction (11-12). Note that the oxidation state of the carbon is changed from 0 to +2.
(11-12)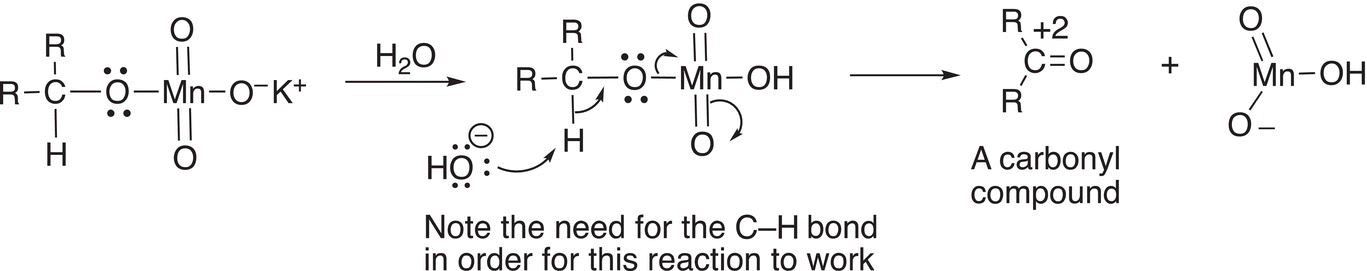
Aldehydes can be oxidized to carboxylic acids by the same reagent, KMnO4, in a basic solution. The first step of the mechanism is shown in Reaction (11-13).
(11-13)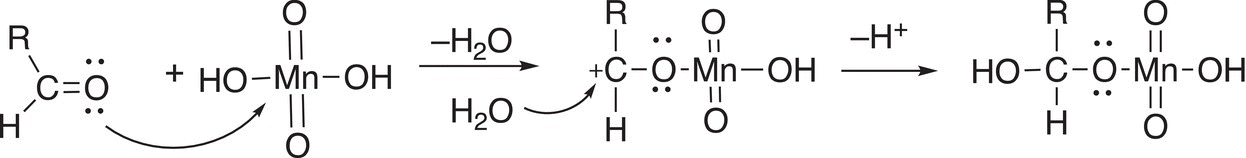
In the final step of the reaction mechanism, the hydrogen is lost to give the oxidized product, as shown in Reaction (11-14).
(11-14)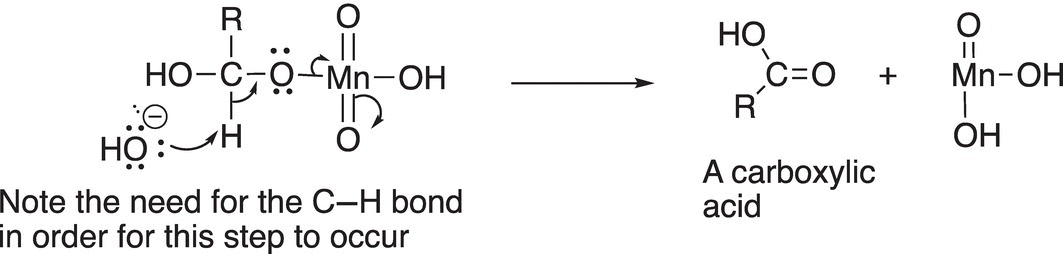
As you can imagine, the oxidation of a primary alcohol gives the aldehyde first, which is oxidized to the carboxylic acid as shown in Reaction (11-15).
(11-15)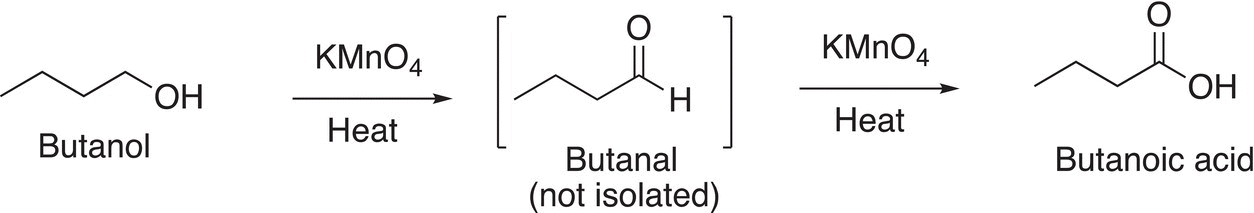
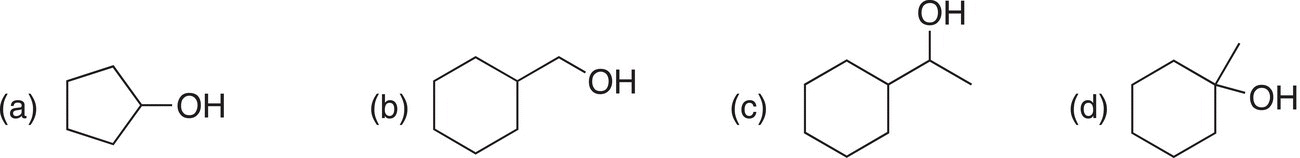
Problem 11.3
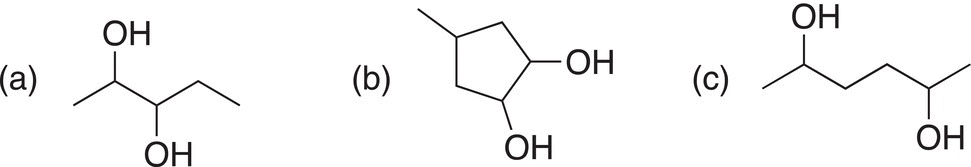
Give the oxidation products of the reaction of the following molecules with KMnO4 in an aqueous solution of KOH.
11.3.2 Oxidation Using Chromic Acid (H2CrO4)
A very similar oxidation as discussed in the previous section can be accomplished using chromic acid. Even in an acidic medium, there is a similar abstraction of the hydrogen bonded to the carbon that has the alcohol functionality as shown in Reaction (11-16).
(11-16)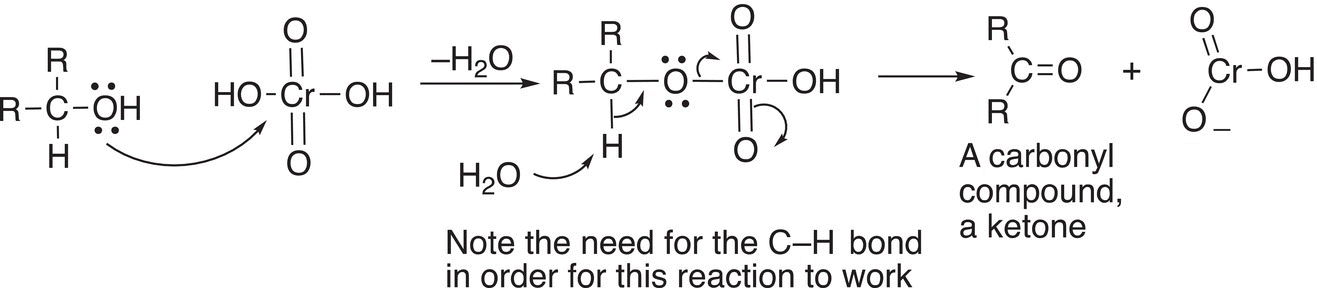
Since there is a hydrogen atom bonded to the carbonyl carbon of aldehydes, these molecules can be oxidized further to give carboxylic acids as shown in the mechanism in Reaction (11-17). The first step is similar to that of the oxidation of alcohols, but instead of the alcohol oxygen attacking the electropositive chromium atom, it is the carbonyl oxygen. A carbocation is created, which is readily attacked by the nucleophilic water to form the intermediate shown in Reaction (11-17).
(11-17)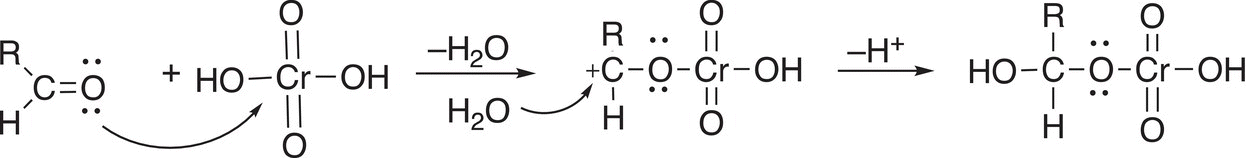
In the next step of the mechanism, water abstracts the proton bonded to the carbonyl carbon to form the carboxylic acid and reduced chromium salt as shown in Reaction (11-18).
(11-18)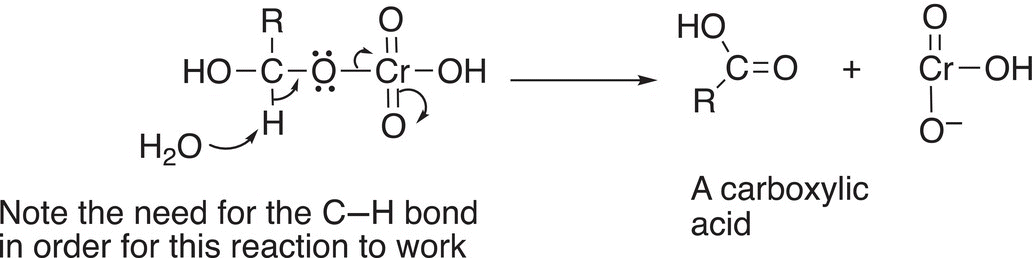
An example of the overall reaction is shown in Reaction (11-19).
(11-19)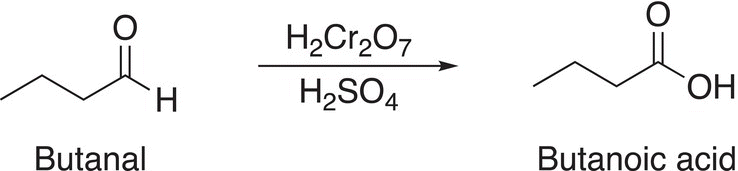
As a result, the oxidation of primary alcohols with a strong oxidizing agent, such as chromic acid or potassium permanganate results in the carboxylic acid, and not the aldehyde. Even though the aldehyde is formed, it is readily oxidized further into the carboxylic acid as shown in Reaction (11-20).
(11-20)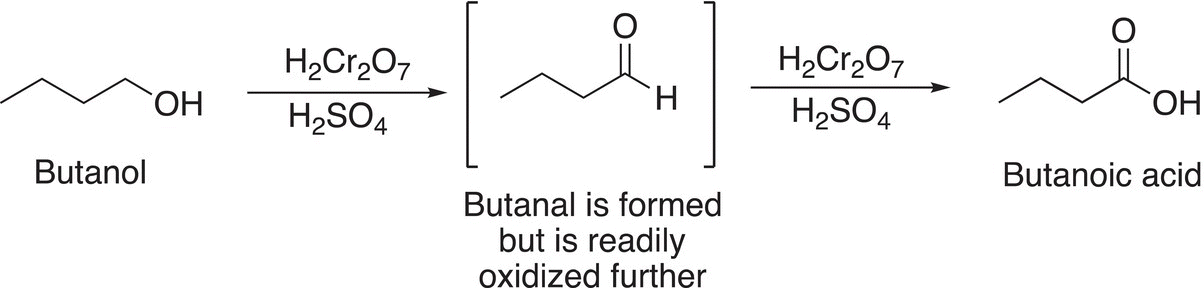
Since tertiary alcohols do not have a hydrogen atom bonded to the carbon of the alcohol functionality, they cannot be oxidized, an example is shown in Reaction (11-21).
(11-21)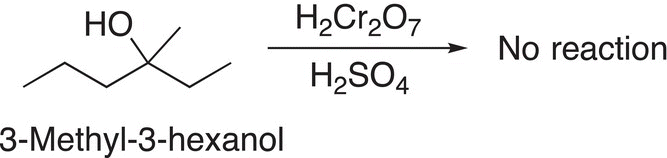
Problem 11.4
Give the oxidized organic product that results from the reaction of each of the following with H2CrO4.
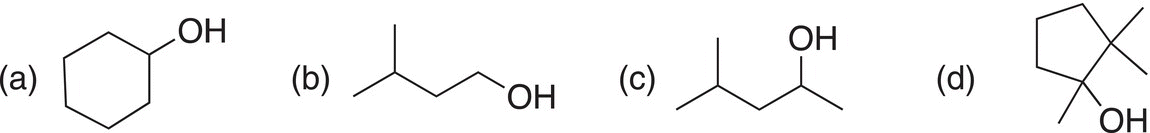
A practical application of this oxidation reaction is that ethanol can be oxidized to acetic acid using a dichromate salt. Dichromate salts are reddish orange in color and once the oxidation has occurred, it is transformed into a green dichromate chromium (III) ion. The reaction is shown in Reaction (11-22). The breath analyzer test, which is used to determine the presence of alcohol, relies on this color change for a positive result based on the reaction given in Reaction (11-22).
(11-22)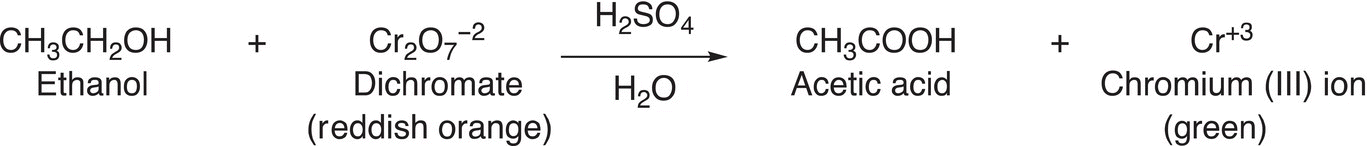
Another variation of this oxidizing agent in which a chromium salt is used is the Jones reagent, which is named after its discoverer by Sir Ewart Jones, an English chemist. The Jones reagent consists of a solution of chromium trioxide in dilute sulfuric acid and acetone and is used to oxidize alcohols to ketones and carboxylic acids, an example is shown in Reaction (11-23).
(11-23)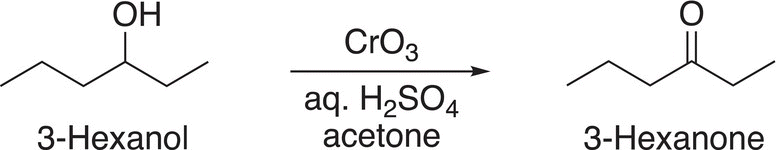
11.3.3 Swern Oxidation
Chromium salts are typically toxic, and extreme care must be exercised in their use. As a result, an alternate set of reagents that is used for oxidation is oxalyl chloride and dimethyl sulfoxide (DMSO); this oxidation was developed by Daniel Swern, an American Chemist, and is now known as the Swern oxidation. The general reaction is shown in Reaction (11-24).
(11-24)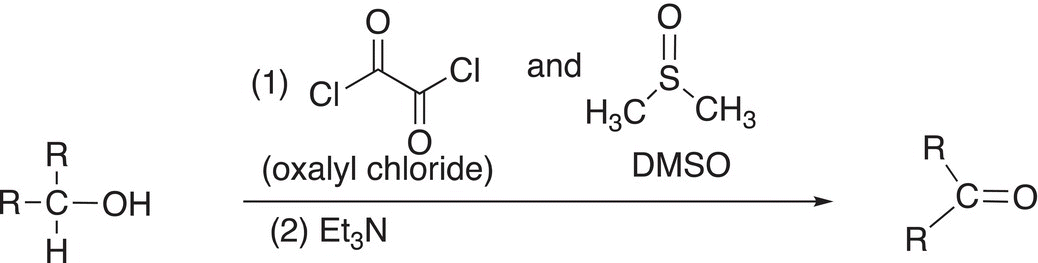
Note again that in order for the oxidation to take place, there must be a hydrogen atom bonded to the carbon of the alcohol group. For the first step of the mechanism, the nucleophilic oxygen of the DMSO attacks the electrophilic carbon of oxalyl chloride to displace a chloride anion followed by the attack of the chloride anion on the electrophilic sulfur atom, as shown in Reaction (11-25).
(11-25)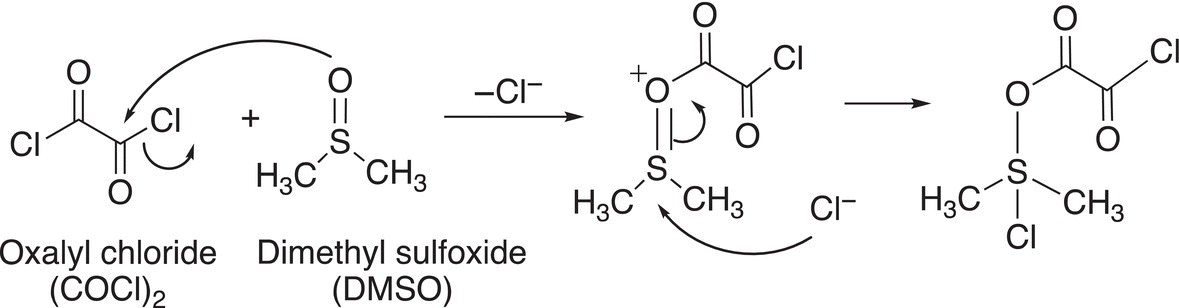
In the next step of the reaction, the intermediate falls apart to release two gases, carbon dioxide and carbon monoxide, and a chlorosulfonium salt, as shown in Reaction (11-26).
(11-26)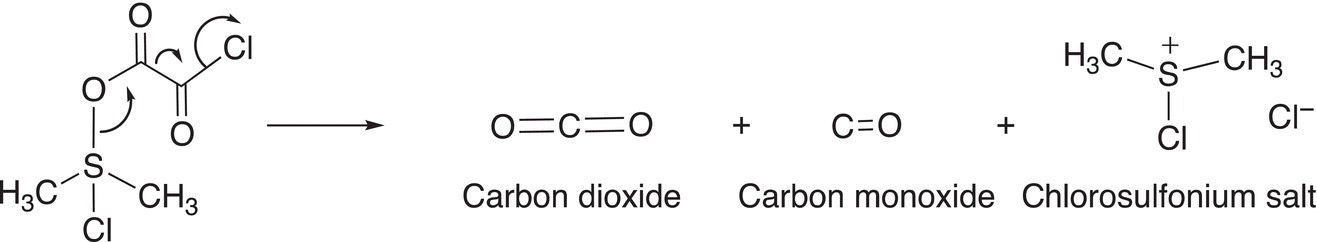
In the next step of the mechanism, the chlorosulfonium salt reacts with the alcohol, as shown in Reaction (11-27) to form an alkylsulfonium salt.
(11-27)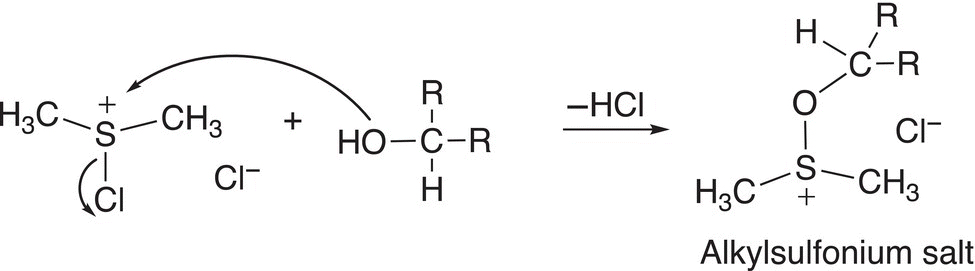
In the next step of the reaction mechanism, the alkylsulfonium salt reacts with the base, triethylamine, to form a ylide, as shown in Reaction (11-28). You will recall that ylides are neutral compounds, in which there is a formal charge of negative charge on a carbon and on the adjacent atom, typically phosphorous, sulfur or nitrogen, there is a formal positive charge.
(11-28)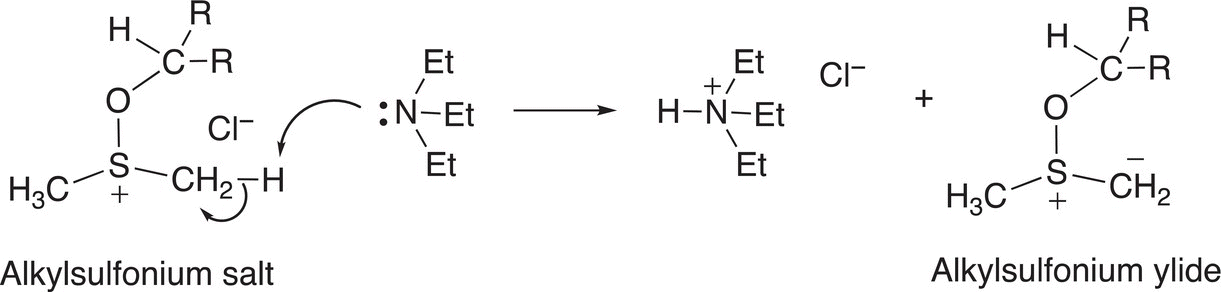
In the next step of the mechanism, the ylide breaks apart to form the oxidized carbonyl compound, as shown in Reaction (11-29).
(11-29)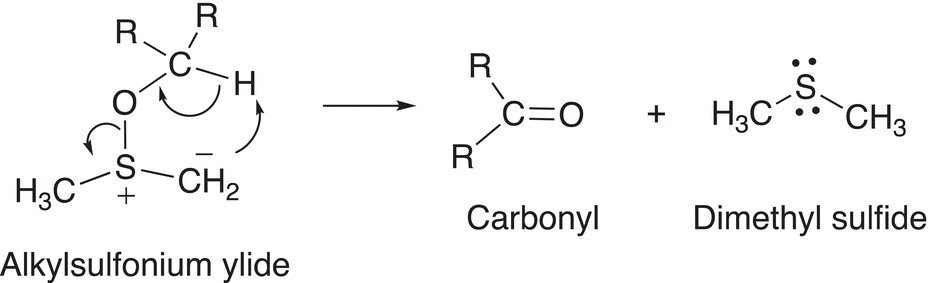
11.3.4 Dess-Martin Oxidation
Another environmentally-friendly method for the oxidation of alcohols is with the use of hypervalent iodine compounds. This reaction, also known as the Des-Martin periodinane (DMP) reaction, derived its name from two American chemists, Daniel Benjamin Dess and James Cullen Martin, who developed this reaction. This reaction is similar to the Swern oxidation in that primary alcohols are oxidized to aldehydes and secondary alcohols are oxidized to ketones; tertiary alcohols are not oxidized as expected. An example of the reaction is shown in Reaction (11-30).
(11-30)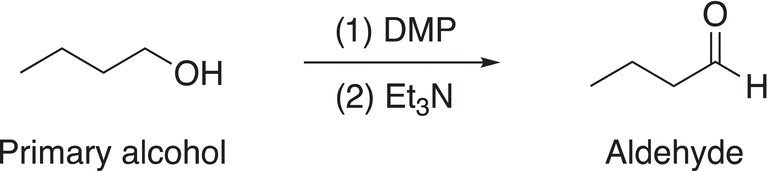
The mechanism involves an attack of the alcohol on the electrophilic iodine of the periodinane compound displacing an acetate anion as shown in Reaction (11-31).
(11-31)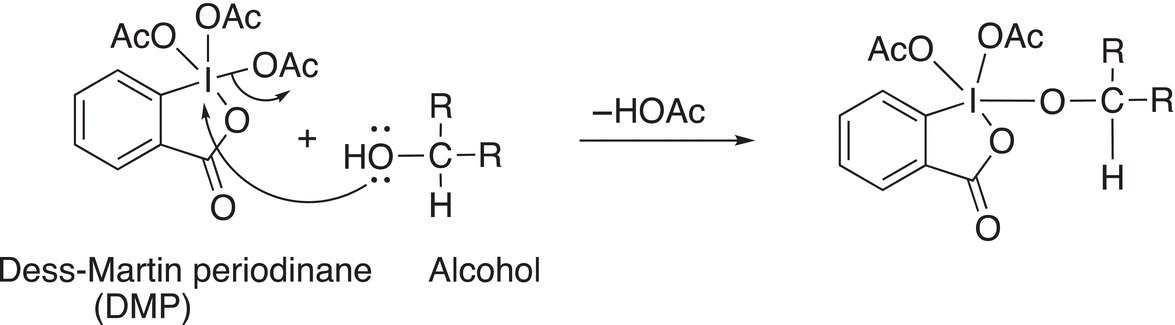
In the next step of the reaction mechanism, the amine base abstracts a proton as shown in Reaction (11-32) to give the oxidized carbonyl compound.
(11-32)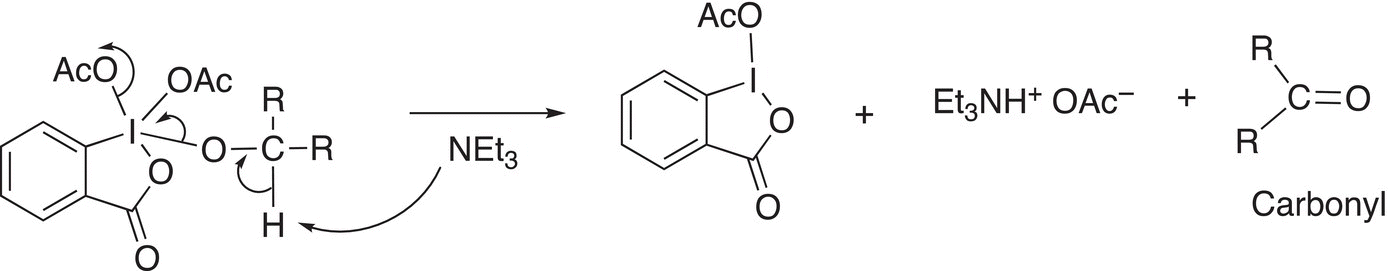
11.3.5 Oxidation Using Pyridinium Chlorochromate
As we have seen from the oxidizing agents discussed thus far, some will oxidize primary alcohols to the aldehyde and will not proceed further to give the carboxylic acid, while other stronger oxidizing agents will oxidize primary alcohols to carboxylic acids. Another mild oxidizing agent results from mixing CrO3 in with HCl and pyridine to give pyridine chlorochromate as shown in Reaction (11-33).
(11-33)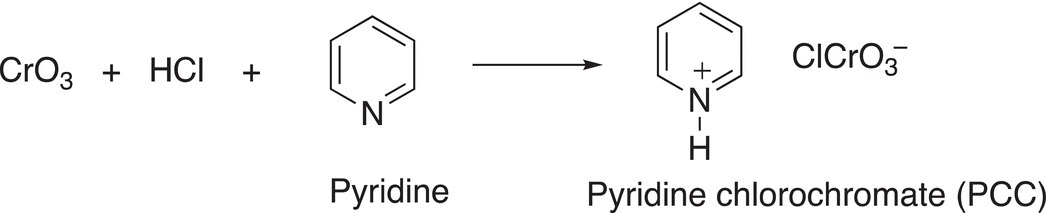
This much milder oxidizing agent is also referred to as PCC and will oxidize primary alcohols just to the aldehyde and not further to give the carboxylic acid, as shown in Reaction (11-34).
(11-34)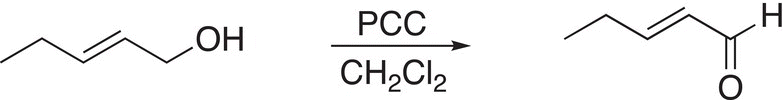
Since it is such a mild oxidizing agent, it will not oxidize other functional groups, such as double bonds, as we will see later in the chapter that stronger oxidizing agents will react with double bonds.
Problem 11.5
i. Give the oxidized products for the following reactions.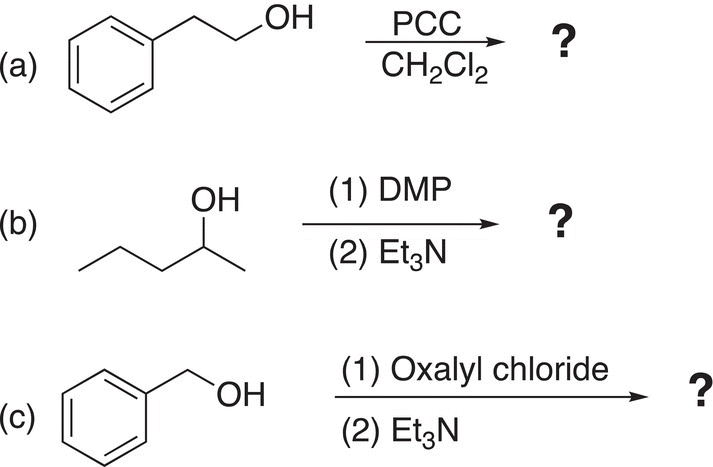
ii. Give the organic reactants to complete the following reactions.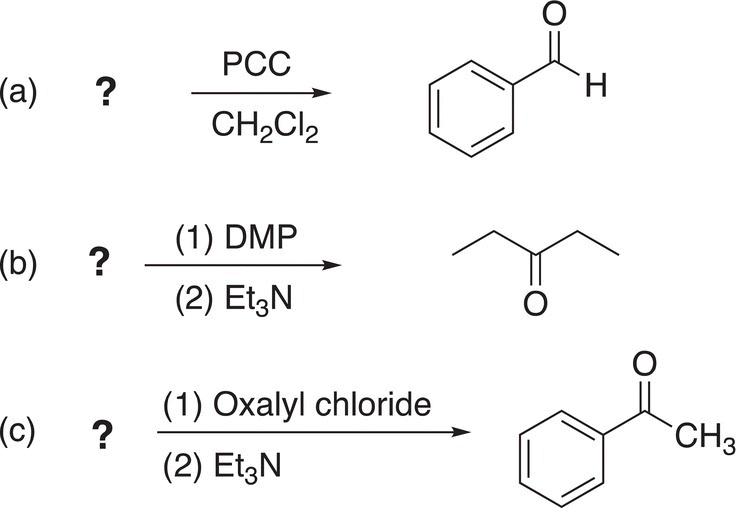
11.3.6 Oxidation Using Silver Ions
The oxidation of aldehydes by silver ions gives an interesting result (a silver mirror), and as a result, this reaction is used as a test for the presence of aldehydes. This test is called the Tollens Test for aldehydes. The general reaction is shown in Reaction (11-35).
(11-35)
Problem 11.6
i. Which of the following compounds would give a positive Tollens test?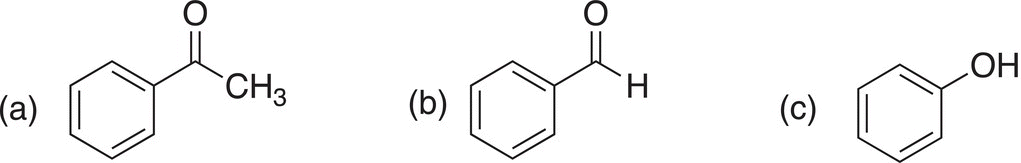
ii. Give the organic reactants that could be used to give the following compounds by an oxidation reaction.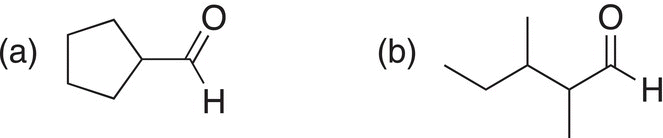
11.3.7 Oxidation Using Nitrous Acid
Nitrous acid is an oxidizing agent and is typically used in organic chemistry to oxidize amines to azide salts, as shown in Reaction (11-36).
(11-36)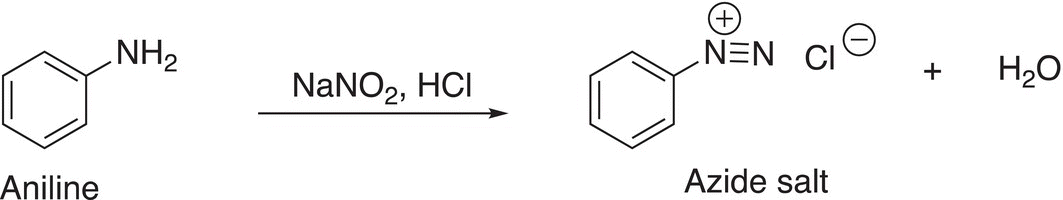
The first step of the mechanism for this reaction involves an attack of the nucleophilic amine functionality on the electrophilic nitrogen of the NO2 group. In the next step, one of the ─OH groups is protonated and leaves as water, as shown below.
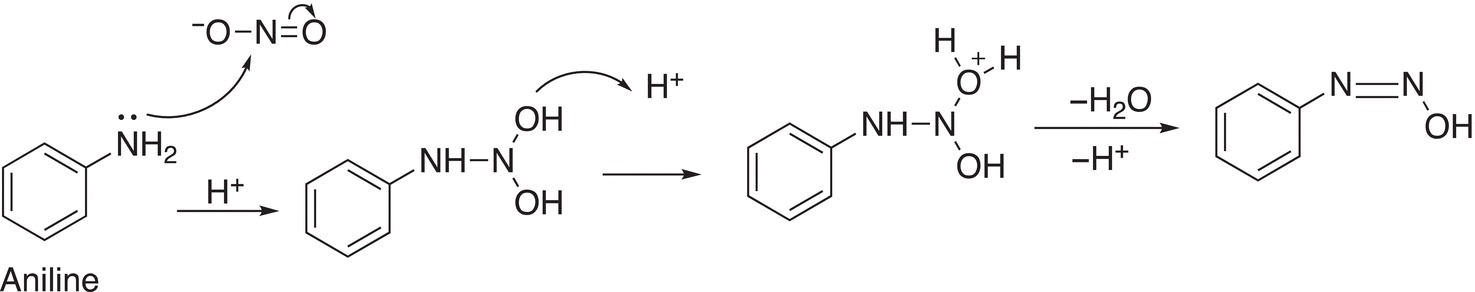
In the next step, protonation of the other ─OH group occurs and it is converted to a good leaving group and leaves as water resulting in the azide salt as shown below.
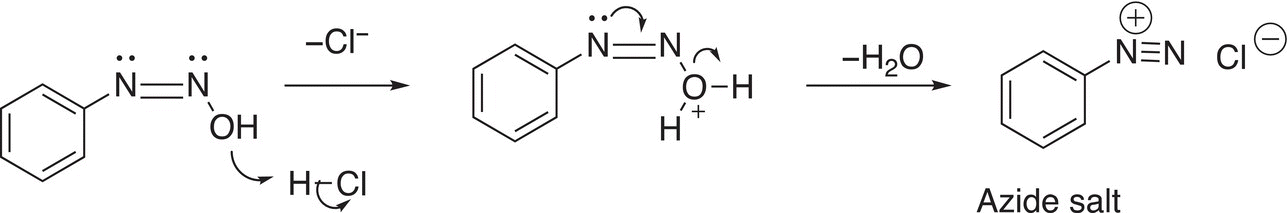
These reactions will be discussed in Chapter 17 on aromaticity, but these reactions are typically used in the preparation of azo dyes. Azo dyes are brightly colored compounds, which are used as dyes for various textiles, particularly cotton, silk, wool, and some synthetic fabrics.
11.3.8 Oxidation Using Periodic Acid
Based on the number of oxygen atoms that are present in periodic acid (HIO4∙2H2O), it is not surprising that it is a strong oxidizing agent. This reagent is used in organic chemistry to cleave 1,2-diols via an oxidation reaction as shown in Reaction (11-37).
(11-37)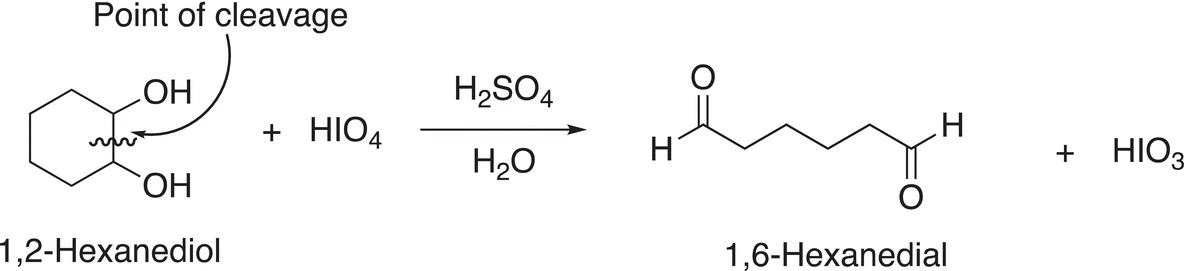
Note that the product for this reaction is a dialdehyde. If a methyl group is bonded to one of the carbons that contains the alcohol functionality, that functionality will be converted into a ketone, as shown in the Reaction (11-38).
(11-38)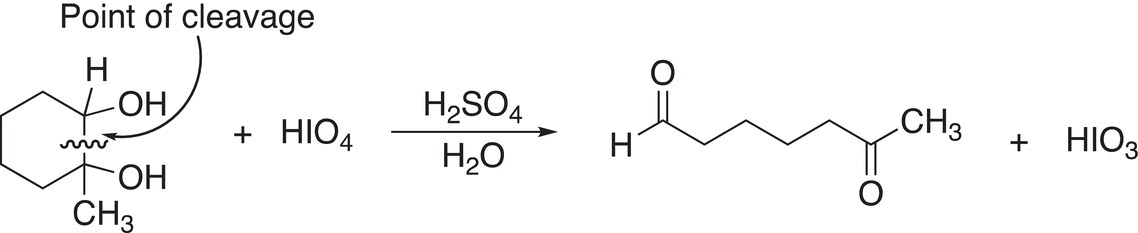
Examination of the mechanism for the reaction will explain the products formed. In the first step, one of the alcohol functionalities bonds to the very electrophilic iodine, after an attack of the other alcohol oxygen and loss of water, a cyclic periodate intermediate is formed as shown in Reaction (11-39).
(11-39)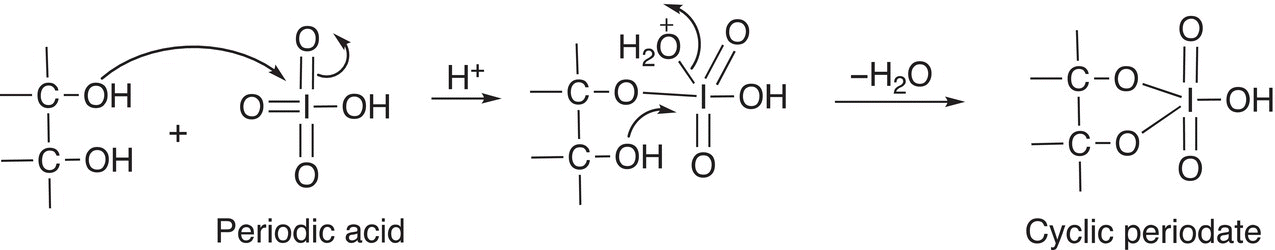
In the next step of the mechanism, the cyclic periodate decomposes with a carbon—carbon bond cleavage to form two carbonyl compounds (or a single compound if the initial diol is cyclic) and iodic acid, as shown in Reaction (11-40).
(11-40)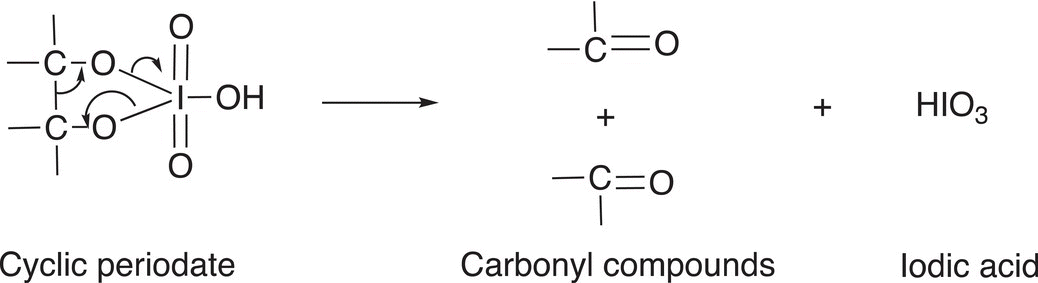
Problem 11.7
Give the organic product(s) that results from the reaction of each of the following molecules with HIO4. If the reaction is not like to occur, indicate no reaction.