Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Oxidation Reactions in Organic Chemistry
11.9 Autooxidation of Ethers and Alkenes
Extreme care must be exercised in the lab when using diethyl ether as a solvent due to the possibility of auto oxidation of ether, which results in an explosive product. Reaction (11-93) gives the products on oxidation with oxygen in the presence of light; the peroxides produced from these reactions are extremely explosive.
(11-93)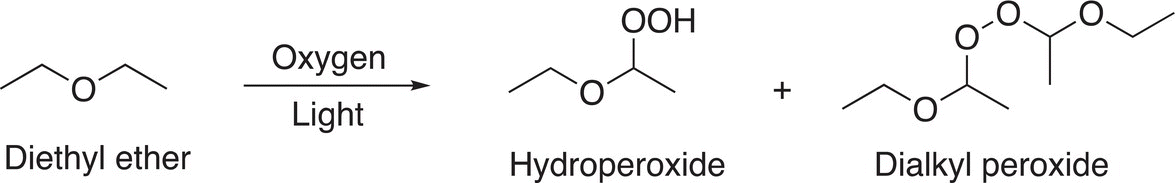
Alkenes can undergo a similar oxidation with oxygen in the presence of light. Cooking oil, which contains unsaturated systems, in the presence of oxygen will undergo autooxidation and this oxidation explains the observation that if a bottle of cooking oil remains unopened for a long period of time, when it is eventually opened, air rushes in to replace oxygen used up due to the oxidation process. The oxygen present in the unopened bottle will be used up due to oxidation of the alkenes present in the oil; and Reaction (11-94) shows an oxidation reaction that is possible.
(11-94)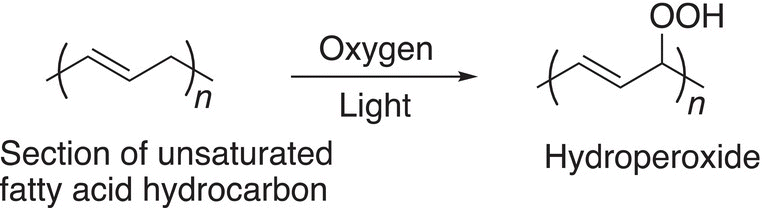
The mechanism for the oxidation of alkenes by oxygen in the presence of light is shown below.
(11-95)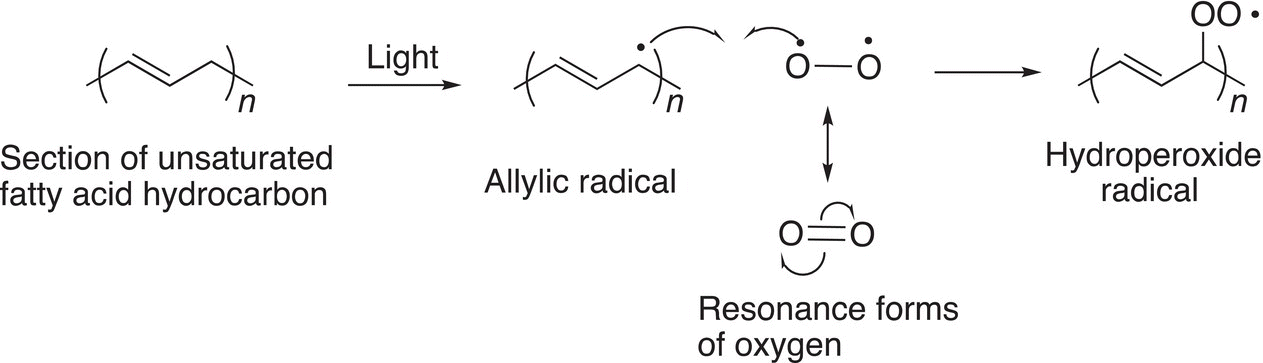
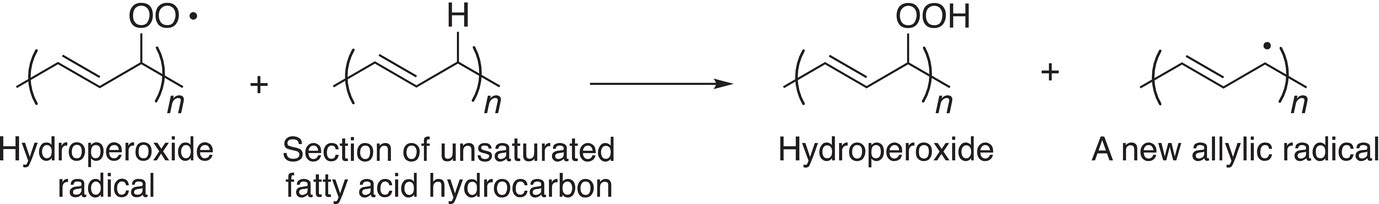
Once the radical is formed, it will react with oxygen to form a hydroperoxide radical, which abstracts a hydrogen atom from another molecule of the alkene to produce a new radical.
(11-96)