Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Aromaticity and Aromatic Substitution Reactions
17.3 Nomenclature of Substituted Benzene
17.3.1 Nomenclature of Monosubstituted Benzenes
The removal of one hydrogen and its substitution by a different atom, or group of atoms, results in a monosubstituted benzene as shown below Reaction (17-2).
(17-2)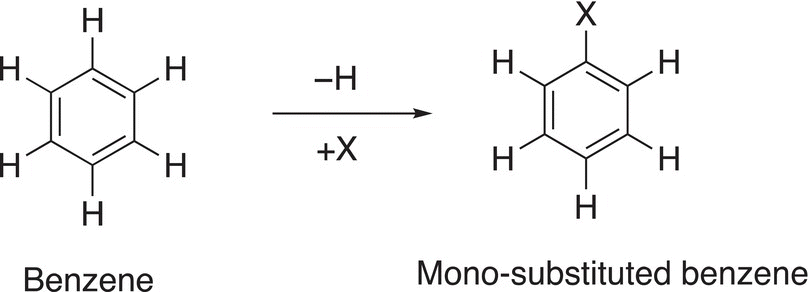
These compounds are named as derivatives of benzene. For example, if X were methyl (CH3), the name is methylbenzene. Other examples of monosubstituted benzenes are shown below.
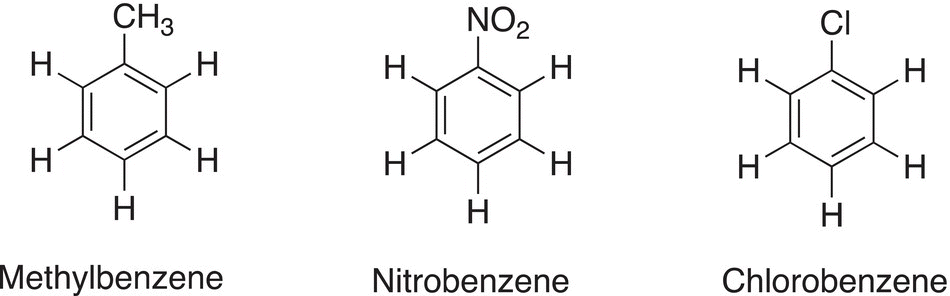
Table 17.1 Names that are used for some monosubstituted benzene molecules.
X |
Common name |
CH3 |
Toluene |
NH2 |
Aniline |
OH |
Phenol |
COOH |
Benzoic acid |
CHO |
Benzaldehyde |
OCH3 |
Anisole |
There are other names that are frequently used if specific substituents are bonded to the benzene ring; in fact, these names are used as root names for the IUPAC system of nomenclature of substituted benzene molecules. Table 17.1 shows the most commonly used names based on different monosubstituents.
Problem 17.2
i. Give the structure of each of the following compounds.
(a) Bromobenzene, (b) hydroxybenzene (phenol), (c) fluorobenzene.
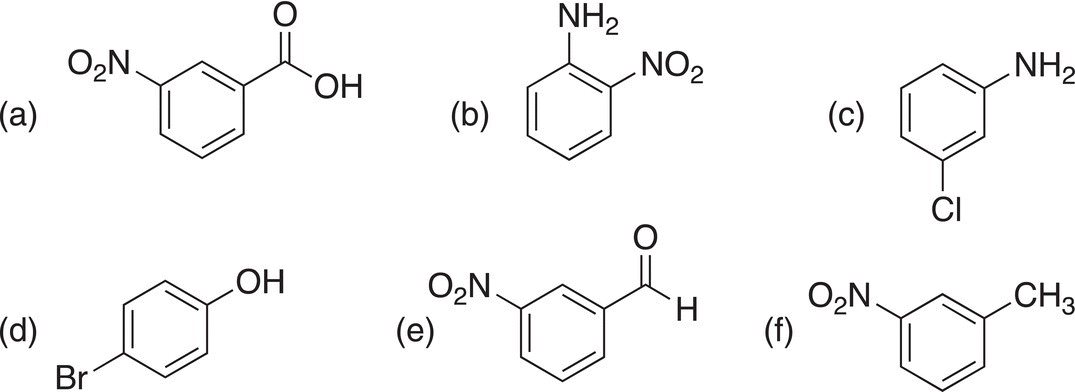
ii. Give the names for the following molecules.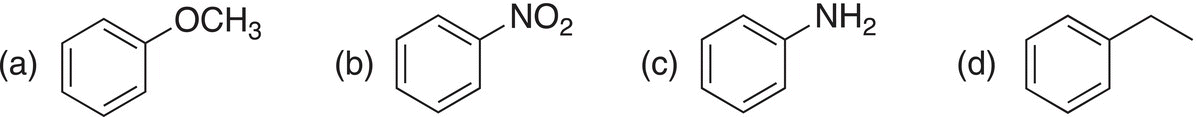
17.3.2 Nomenclature of Di-Substituted Benzenes
If there are two substituents bonded to the benzene ring (sometimes referred to as the phenyl ring), the exact relationship between the substituents on the ring must be specified. There is the possibility of different isomers based on the relationship of the groups on the phenyl ring. Thus, if benzene has two methyl groups bonded to the ring, there is the possibility of three different isomers — different molecules as shown in Figure 17.6.
In naming such disubstituted benzene compounds, as expected, one substituent must take priority and it is assigned #1 and priority is given based on the alphabet. The other substituent gets an assigned number based on its relationship to that group that is in position number 1. Examples are shown in Figure 17.7.
Often times there is an easily recognizable portion of the disubstituted benzene that has a name as shown in Table 17.1. In that case, the name of the disubstituted benzene is based on the root name shown in Table 17.1. Note that the position of the group that is used to determine the name of the compound gets number 1. Examples are shown in Figure 17.8.
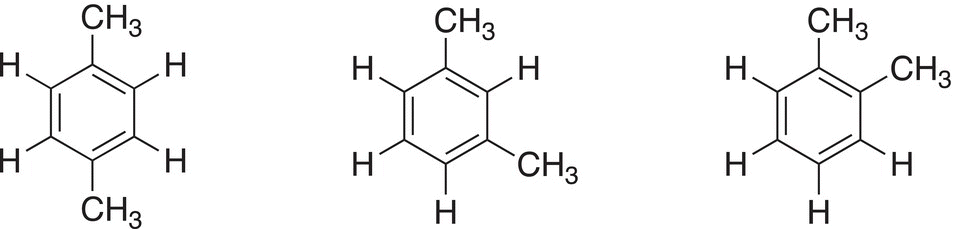
Figure 17.6 Different isomers of dimethylbenzenes.
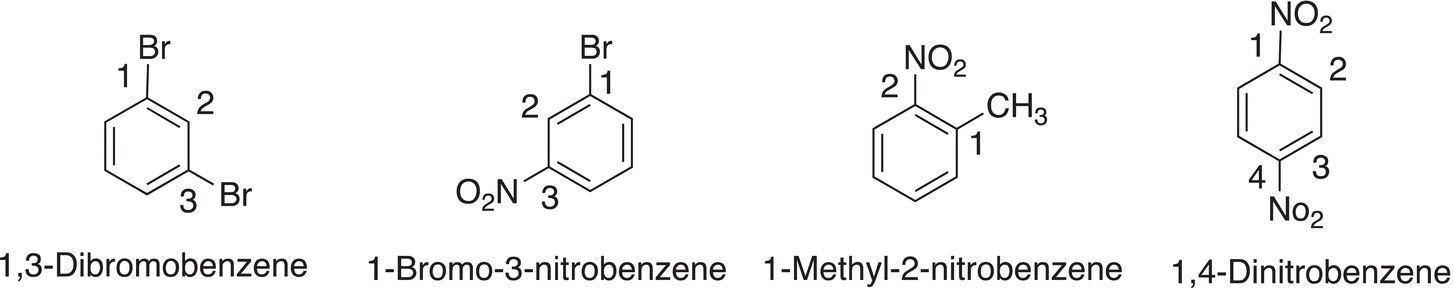
Figure 17.7 Examples of disubstituted benzene.
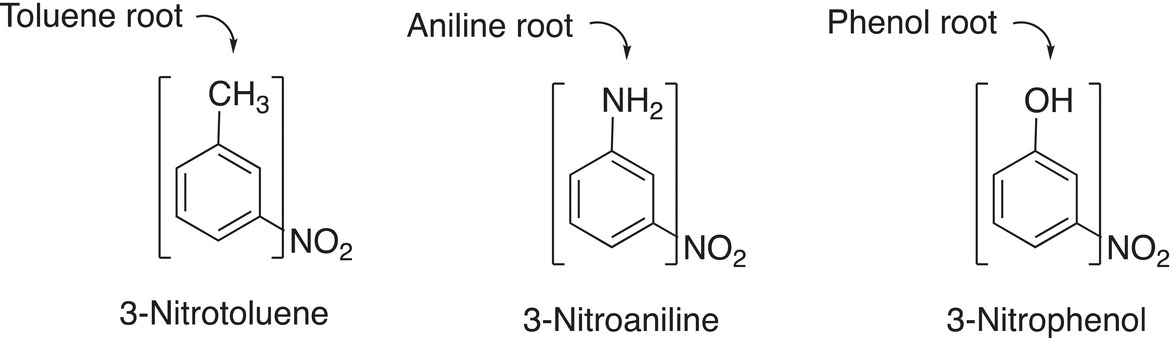
Figure 17.8 Examples of disubstituted benzenes.
Instead of using numbers in the nomenclature of disubstituted compounds, there is another method of naming these molecules. The nomenclature is accomplished by assigning common terms to the numbered relationships mentioned above. The common terms for the relationships are shown in Figure 17.9.
Examples using this system of nomenclature are shown in Figure 17.10.
Problem 17.3
i. Give the structures for each of the following compounds.
(a) meta-Bromobenzaldehyde, (b) p-Chlorophenol,(c) 2-Fluoroaniline,(d) o-Hydroxybenzoic acid, and (e) 4-Nitrophenol.
ii. Give the names of the following molecules.
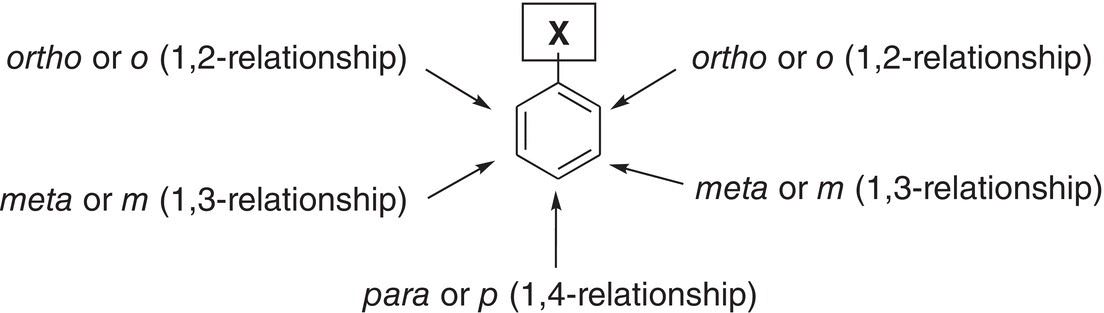
Figure 17.9 Common representations for the numbered relationships used in the naming of disubstituted benzenes.
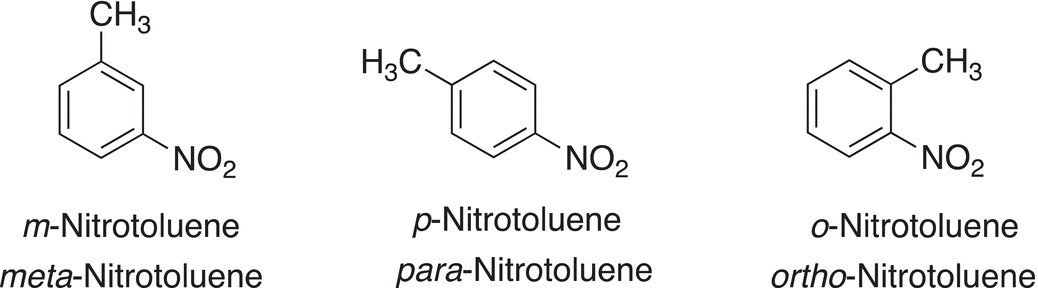
Figure 17.10 Examples of common names of selected disubstituted benzenes.