Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Aromaticity and Aromatic Substitution Reactions
17.8 Applications — Synthesis of Substituted Benzene Compounds
As we have seen at the beginning of the chapter, there are numerous useful compounds that are substituted benzenes. In this section, we will utilize the reactions studied earlier to convert benzene into various substituted benzene compounds. Since benzene is readily available from petroleum, it is a good starting compound from which a host of substituted benzene compounds can be made. In the first example, the oxidation of a CH group directly bonded to a benzene ring (also known as a benzylic CH group) by a strong oxidizing agent is shown in Reaction (17-50).
(17-50)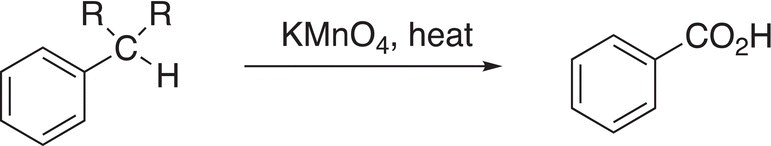
Note that this oxidation converts a benzylic CH into a carboxylic acid group. Also note that this reaction essentially converts an alkyl o,p-director to a meta-director, as shown in the reaction in Reaction (17-51).
(17-51)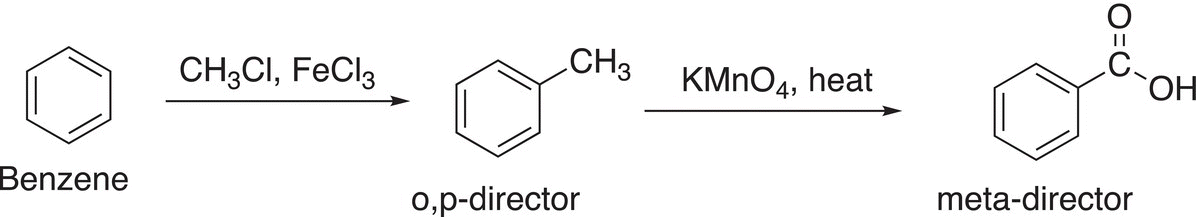
This strategy can be used to synthesize slightly different compounds, as illustrated in Reactions (17-52) and (17-53).
(17-52)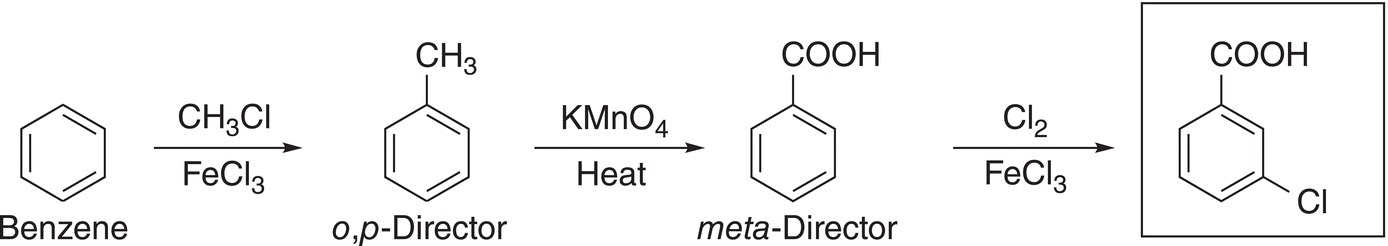
(17-53)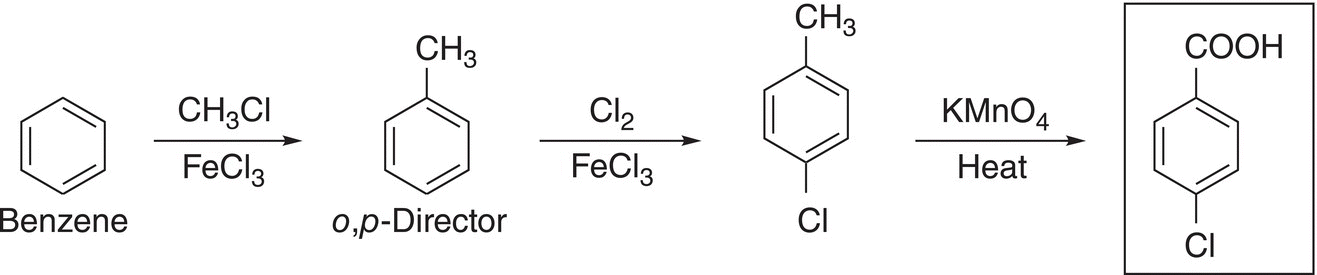
Even though the same reactions and starting compound are used for both Reactions (17-52) and (17-53), the final products are different; the only difference is the sequence of these reactions. For Reaction (17-52), the methyl group is converted to the carboxylic acid group, which makes it a meta-director for the incoming chlorine. For Reaction (17-53), the methyl group is used as a o,p-director for the incoming chlorine.
A similar strategy is illustrated for the sequence of reactions in Reaction (17-54).
(17-54)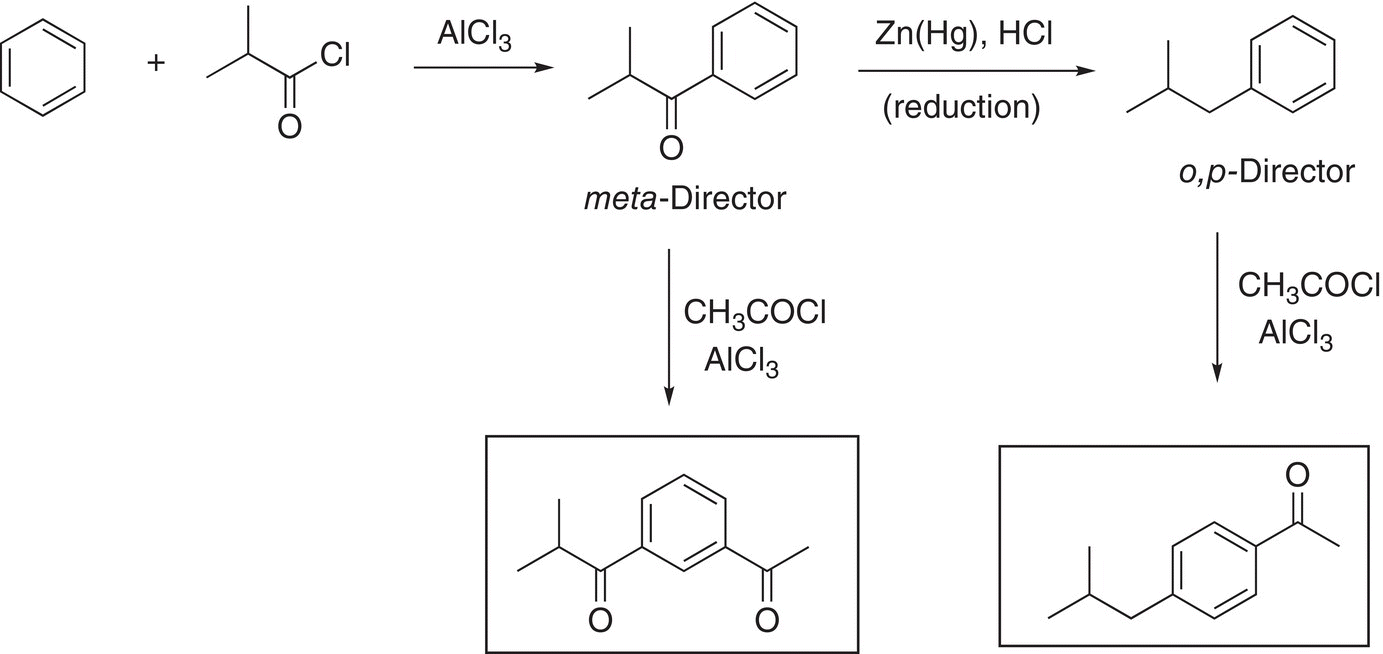
Again, the same starting compound, benzene and essentially the same reactions are used in the reaction scheme above, yet the final products are different just based on a slight difference in the sequence of reactions. One of these reaction sequences can used to synthesize an important compound, ibuprofen, and is outlined in the reaction scheme in Reactions (17-55) and (17-56).
(17-55)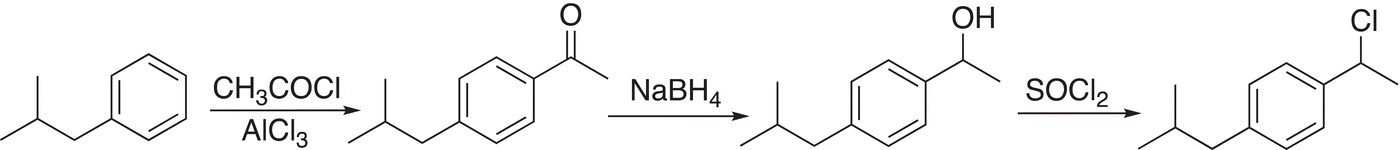
Note that since the relationship between the groups in our target molecule, ibuprofen, is para, the reaction selected from Reaction (17-54) is the reaction shown to the extreme top right. For the reaction sequence given in Reaction (17-55), reduction of the carbonyl gives a secondary alcohol, which can be converted to a chloride using thionyl chloride (SOCl2).
(17-56)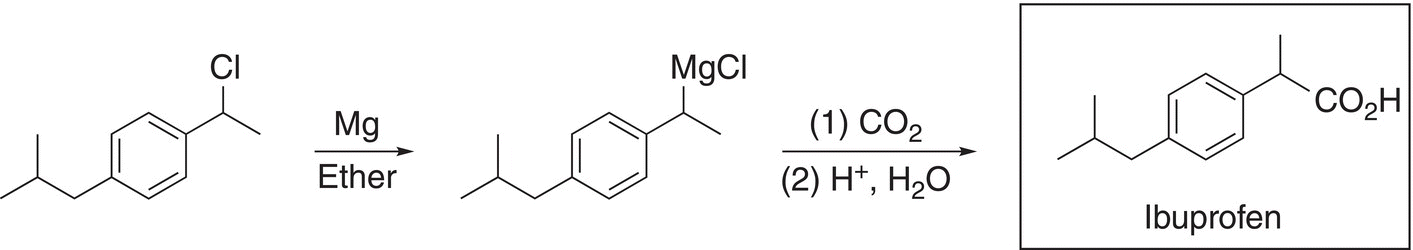
The chlorine functionality can be converted to an organometallic reagent using a Grignard reaction and the Grignard reagent used to reduce carbon dioxide gives the product, ibuprofen, after acidic hydrolysis.
Another example of a similar strategy of introducing a specific group onto a benzene ring and converting it to a different director is shown in the example in Reaction (17-57). The reduction of a nitro group to form an amine is an important reaction, as shown below in Reaction (17-57).
(17-57)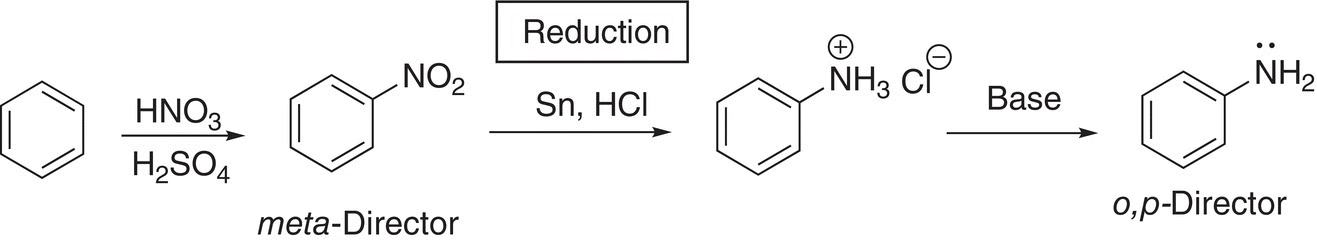
Note for this set of reactions, the nitro group, which is a meta-director is converted to a o,p-director. We can take advantage of this in making different substituted benzenes, as shown in Reaction (17-58).
(17-58)
Since the nitro group is a meta-director, the incoming methyl group will be directed to the meta position of the benzene ring. Once the reduction is accomplished, additional reactions can be accomplished as shown in Reaction (17-59).
(17-59)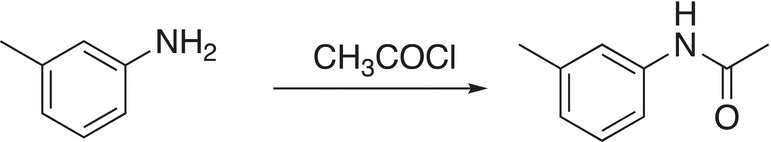
Problem 17.10
Show how to carry out the following transformation.