Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Synthetic Polymers and Biopolymers
20.10 Synthesis of α-Amino Acids
20.10.1 Synthesis of α-Amino Acids Using the Strecker Synthesis
A method to synthesize amino acids was developed by a German chemist, Adolph Strecker, and involves an addition reaction to an aldehyde, followed by a hydrolysis reaction. The general synthetic route is shown in Reaction 20-16.
(20-16)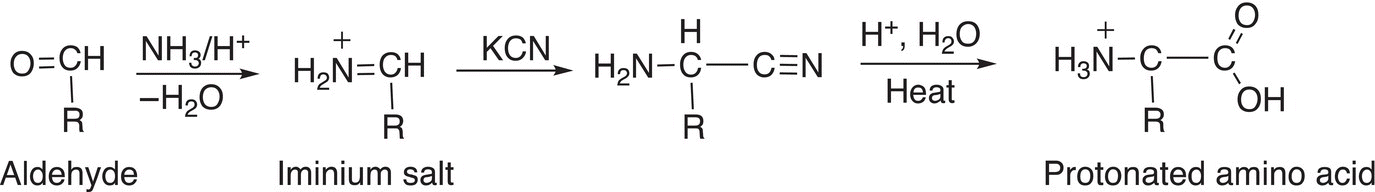
We have seen the type of reactions used in this synthesis throughout our course. The first reaction is an addition—elimination reaction that involves the addition of the nucleophilic ammonia to the electrophilic carbon of an aldehyde, followed by the elimination of water to form an iminium salt. In the next step of the reaction sequence, which is an addition reaction, the nucleophilic nitrile is added to the iminium carbon and the proton is added to the electronegative oxygen to form an amino nitrile. In the last step of the reaction sequence, the nitrile is hydrolyzed under acidic conditions to form the protonated amino acid. Note this sequence of reactions is not stereospecific, and a racemic mixture of the amino acid results.
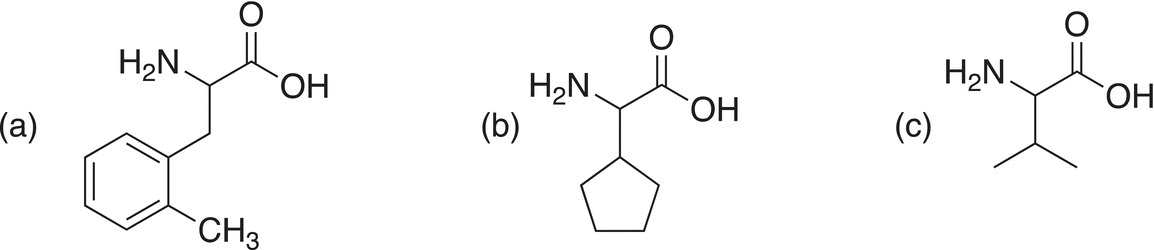
Problem 20.6
Show how to make the following amino acids using the Strecker synthesis.
20.10.2 Synthesis of α-Amino Acids Using Reductive Amination
The synthesis of amino acids using reductive amination involves the reduction of an imine carboxylic acid to form the amino acid, as shown in Reaction (20-17).
(20-17)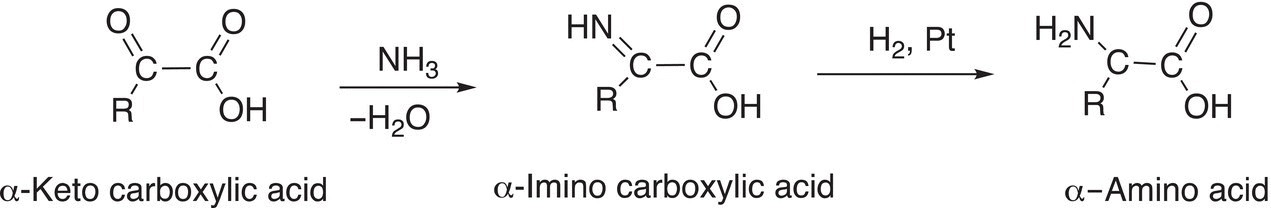
The first step of the reaction involves an addition-elimination reaction, in which ammonia adds, with the accompanying loss of water, to the keto functionality of the α-keto carboxylic acid starting compound. α-Keto carboxylic acids, such as pyruvic acids, play an important role in biology. The second step involves a reduction reaction, in which hydrogen gas reduces the imine to the amine.
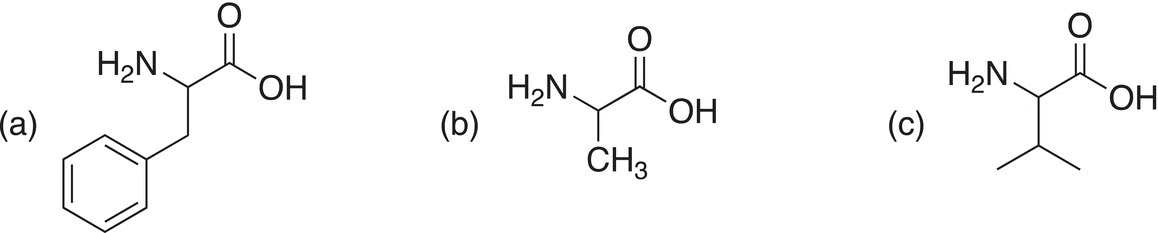
Problem 20.7
Starting with any α-keto carboxylic acid, show how to make the following amino acids using reductive amination.
20.10.3 Synthesis of α-Amino Acids Using Hell Volhard Zelinsky Reaction
This reaction utilizes the reactivity of a hydrogen on the α-carbon of a carboxylic acid. The α-hydrogen is first changed into a good leaving group, in this case, a bromide to form a α-bromocarboxylic acid. The reaction of an α-bromocarboxylic acid with a nucleophilic ammonia results in a substitution reaction in which the ammonia replaces the bromide to result in an α-amino acid as shown in Reaction (20-18).
(20-18)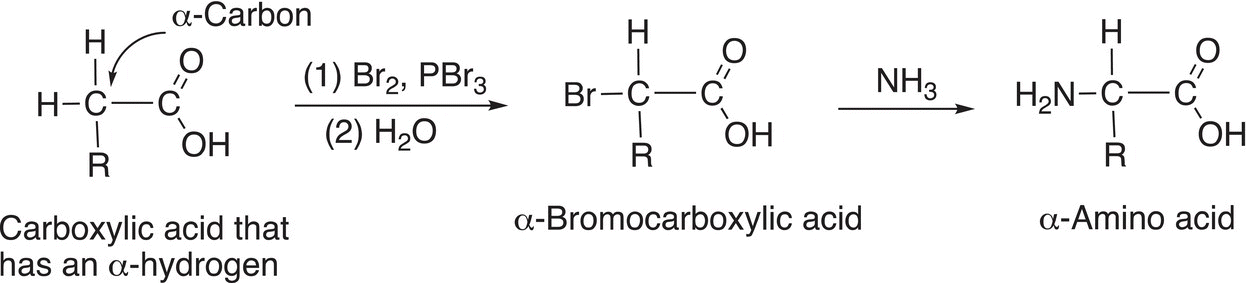
Problem 20.8
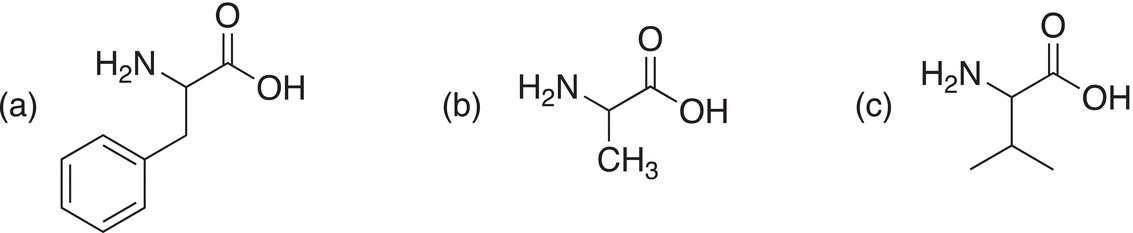
Show how to make the following amino acids using the Hell—Volhard—Zelinsky Reaction.
20.10.4 Synthesis of α-Amino Acids Using the Gabriel Malolic Ester Synthesis
This synthesis takes advantage of introducing an amino group as a primary amine using phthalimide into a molecule that can be converted into an amino acid through a series of reactions as outlined in Reactions (20-19) through (20-21). The first step of the reaction sequence is a Gabriel substitution reaction in which a phthalimide salt reacts with α-bromo-substituted malonic ester by a substitution reaction to form a phthalimide-substituted ester.
(20-19)α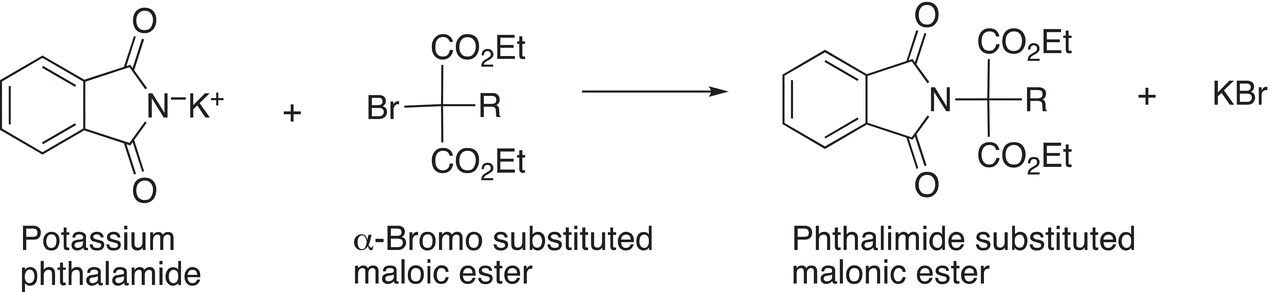
In the next step, the phthalimide substituted malonic ester reacts with hydrazine to liberate the amino substituted malonic ester as shown in Reaction (20-20).
(20-20)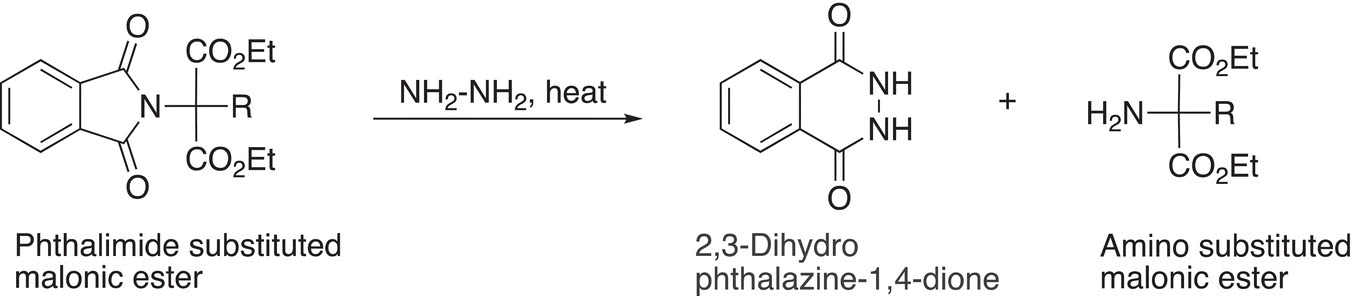
In the last step of the synthesis, the amino substituted malonic ester is hydrolyzed to liberate the amino acid as shown in Reaction (20-21).
(20-21)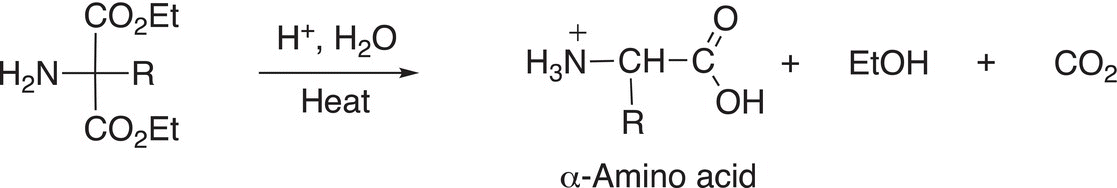
Problem 20.9
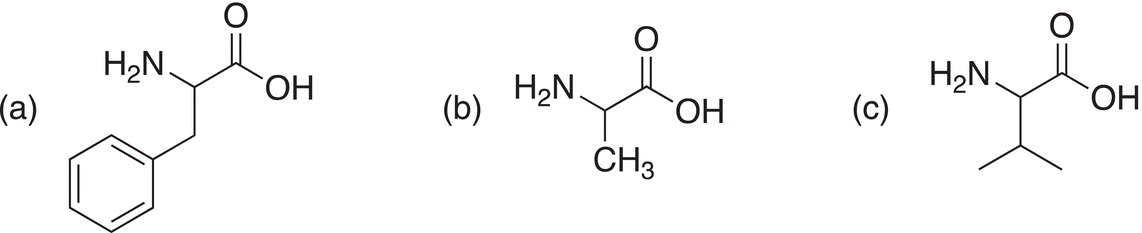
Show how to make the following amino acids using the Gabriel malonic ester synthesis reaction.