Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Alkanes, Cycloalkanes, and Alkenes: Isomers, Conformations, and Stabilities
4.7 Stability of Alkenes
The same type of analysis can be used to determine the relative stability of different structural isomers of alkenes. Consider the hydrogenation of the two alkenes shown in Reactions (4-1) and (4-2). Since both reactions give the same product (2-methylbutane), a comparison of relative energies of the reactants can be carried out by a similar approach as demonstrated with the combustion of alkanes. For the alkene that has the terminal double bond (Reaction 4-1), more heat is released to give the same product, compared to that of the same reaction for the alkene that has an internal double bond (Reaction 4-2).
(4-1)−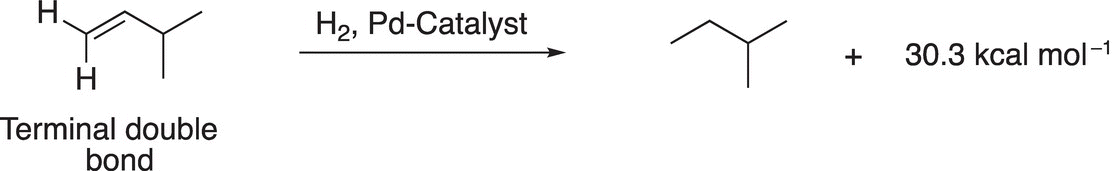
(4-2)−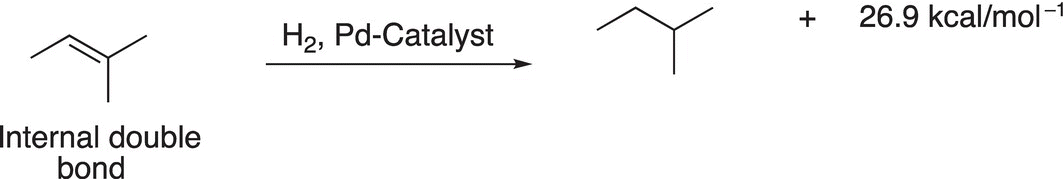
This concept can be represented using an energy profile diagram as shown in Figure 4.28.
Thus, it can be concluded that 2-methyl-2-butene is more stable than 3-methyl-1-butene, and an alkene that has a double bond in a terminal position is less stable than a similar alkene that has the double bond internally.
A similar analysis can be carried out to determine the relative stabilities of cis and trans geometric isomers of alkenes as shown in Reactions (4-3) and (4-4).
(4-3)−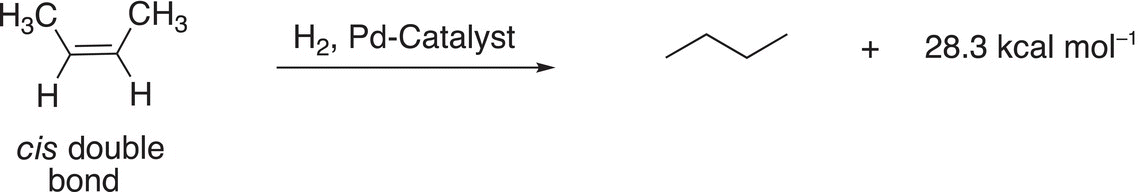
(4-4)−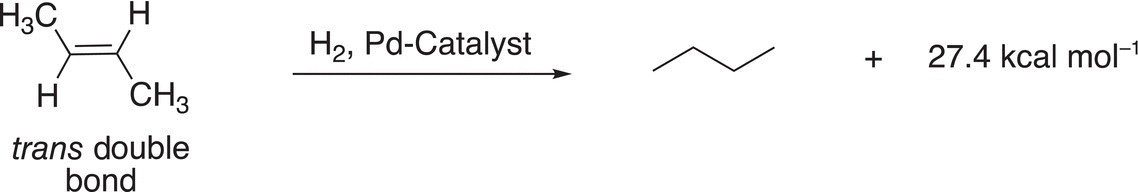
Note that for these reactions, more heat is liberated for the cis butene (Reaction 4-3) and less heat is liberated from trans butene (Reaction 4-4). The energy profile for these reactions is shown in Figure 4.29.
∆−∆−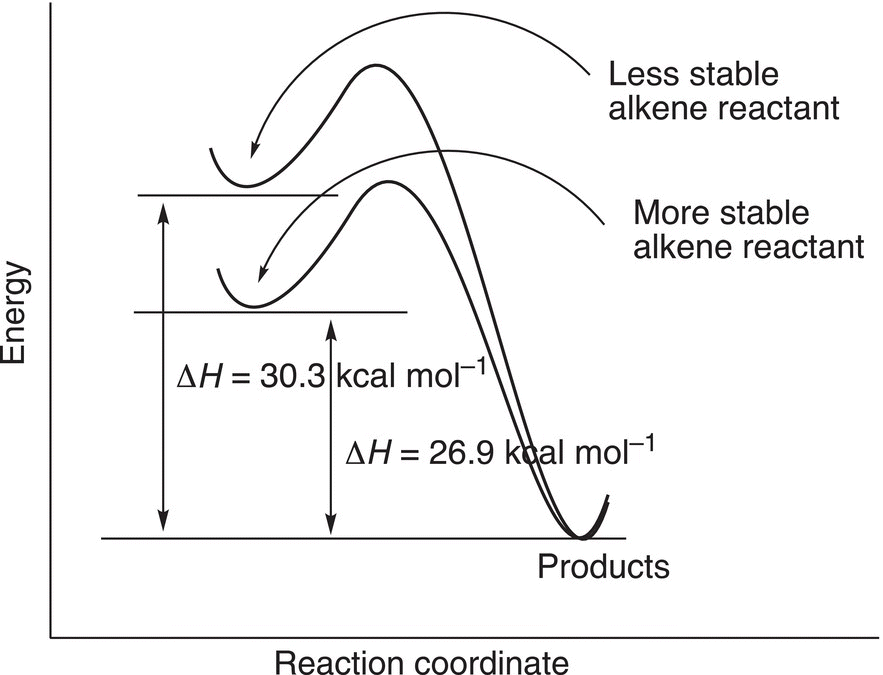
Figure 4.28 Reaction profile for the catalytic hydrogenation of 3-methyl-1-butene and 2-methyl-2-butene.
∆−∆−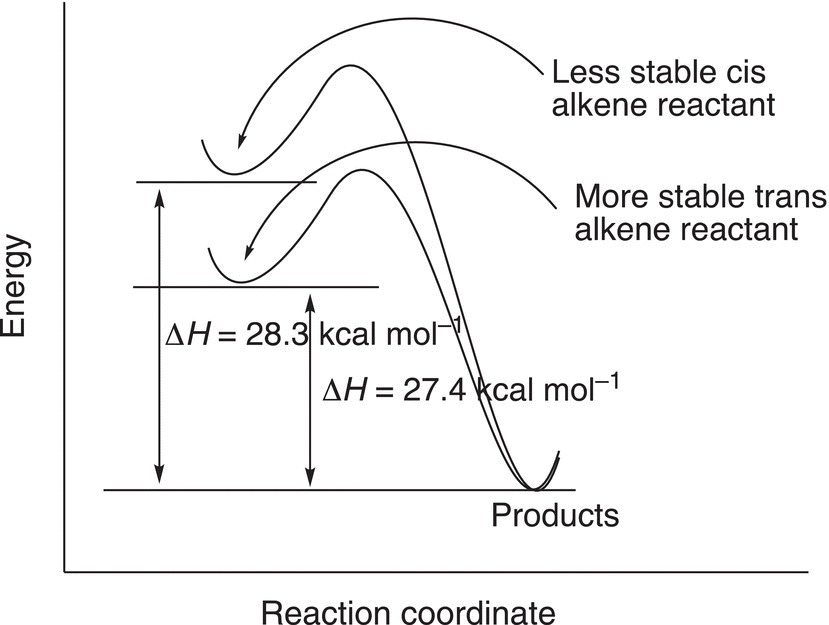
Figure 4.29 Reaction profile for the catalytic hydrogenation of cis-2-butene and trans-2-butene.
Based on these results, it can be concluded that (E)-2-butene is more stable than (Z)-2 butene and that trans alkenes are more stable that cis alkenes.