Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Stereochemistry
5.7 Resolution of Enantiomers
As demonstrated earlier in the chapter, pure enantiomeric compounds are typically needed for specific biological activities; purity of different enantiomeric compounds is of special importance to the pharmaceutical industry. Most of the reactions that will be discussed in later chapters give a mixture of enantiomers. Thus, it is very important to have experimental methods, which can be used to separate a mixture of enantiomers. For a mixture of enantiomers, one strategy that is used is to convert both enantiomers of the mixture into other compounds, such as diastereomers, which have different properties. A mixture of diastereomers can be separated based on differences in their physical properties. Once the separation has occurred, each diastereomer can be converted back to the original pure enantiomer. Figure 5.14 shows a reaction scheme, which can be used to separate enantiomers of 2-methylbutanoic acid.
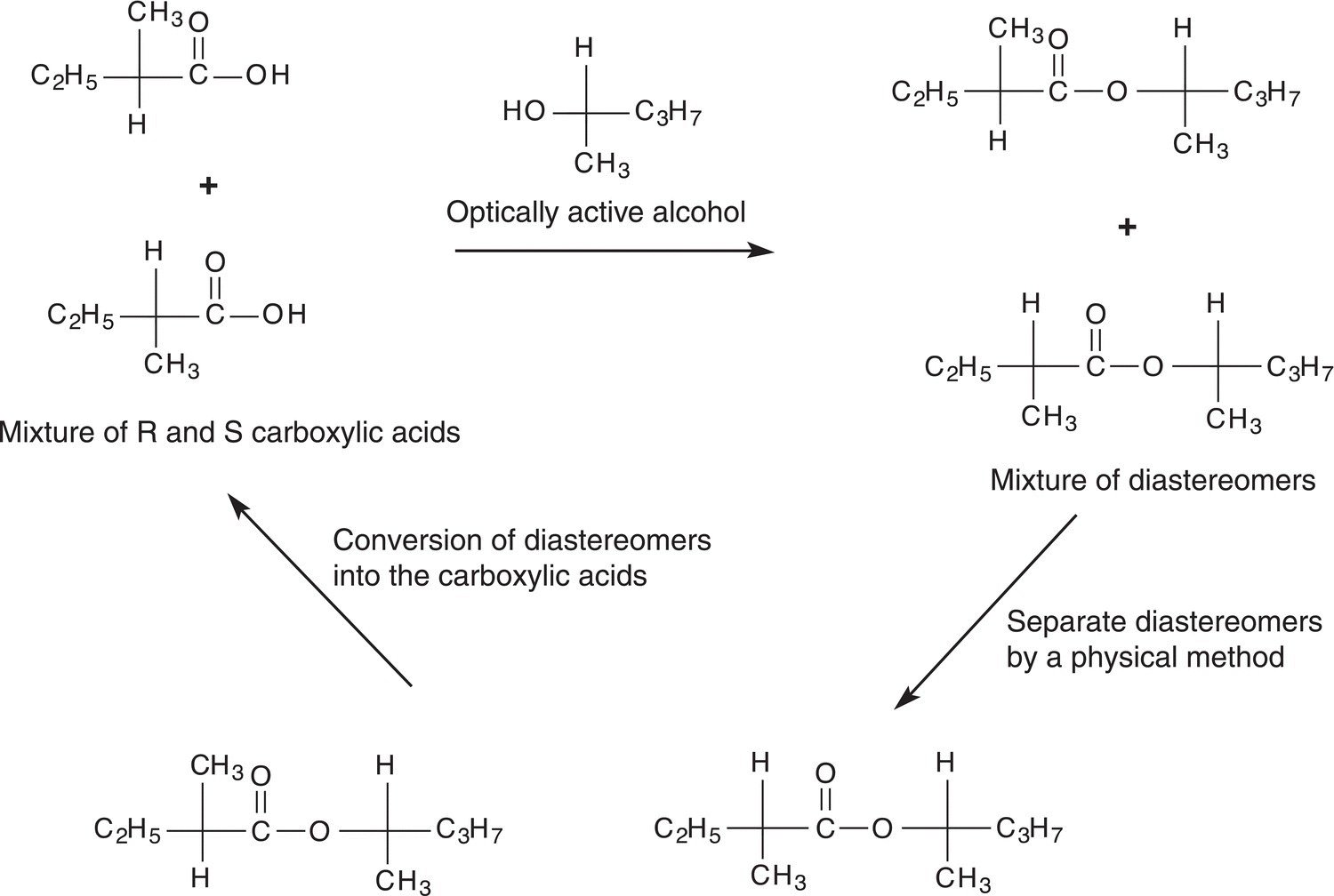
Figure 5.14 An experimental method for the separation of a mixture of enantiomers of 2-methylbutanoic acid.
The first reaction involves the conversion of the enantiomeric mixture of carboxylic acids into the diastereomers in which an optically active alcohol, (R)-2-pentanol, is used. Since the products of this reaction are diastereomers, they can be separated by a physical method such as chromatography. Once the separation has been accomplished, each diastereomer can be converted back into the carboxylic acid. In this case, the reaction is a hydrolysis reaction, which converts the ester into the original reactants, the carboxylic acid and alcohol, and the carboxylic acid is purified from the alcohol, typically by crystallization or distillation depending on the boiling and melting points of the acid. Shown in Figure 5.15 is a schematic illustration of the experimental method used for this purification.
An alternative method of separating enantiomeric mixtures is to pass the mixture of enantiomers through a column that has optically active packing materials. With the appropriate choice of optically active column, there is an attraction between the enantiomers and the optically active packing material of the column, resulting in a diastereomeric complex. Since the properties, including polarity of these diastereomeric complexes, are different, they will move through the column at different rates and hence separated. This technique is typically done using chiral columns with a liquid passing through at a high pressure, this technique is known as high-pressure liquid chromatography (HPLC). Chiral packing materials typically include polysaccharides.
In organic synthesis, one of the major goals is the synthesis of optically pure compounds. As mentioned earlier, most reactions give a mixture of enantiomers, but if specific catalysts are used, a higher percentage of one enantiomer can be achieved over the other enantiomer. To determine the effectiveness of such reactions, the term percent enantiomeric (%ee) excess is used and is defined by Eq. (5-2).
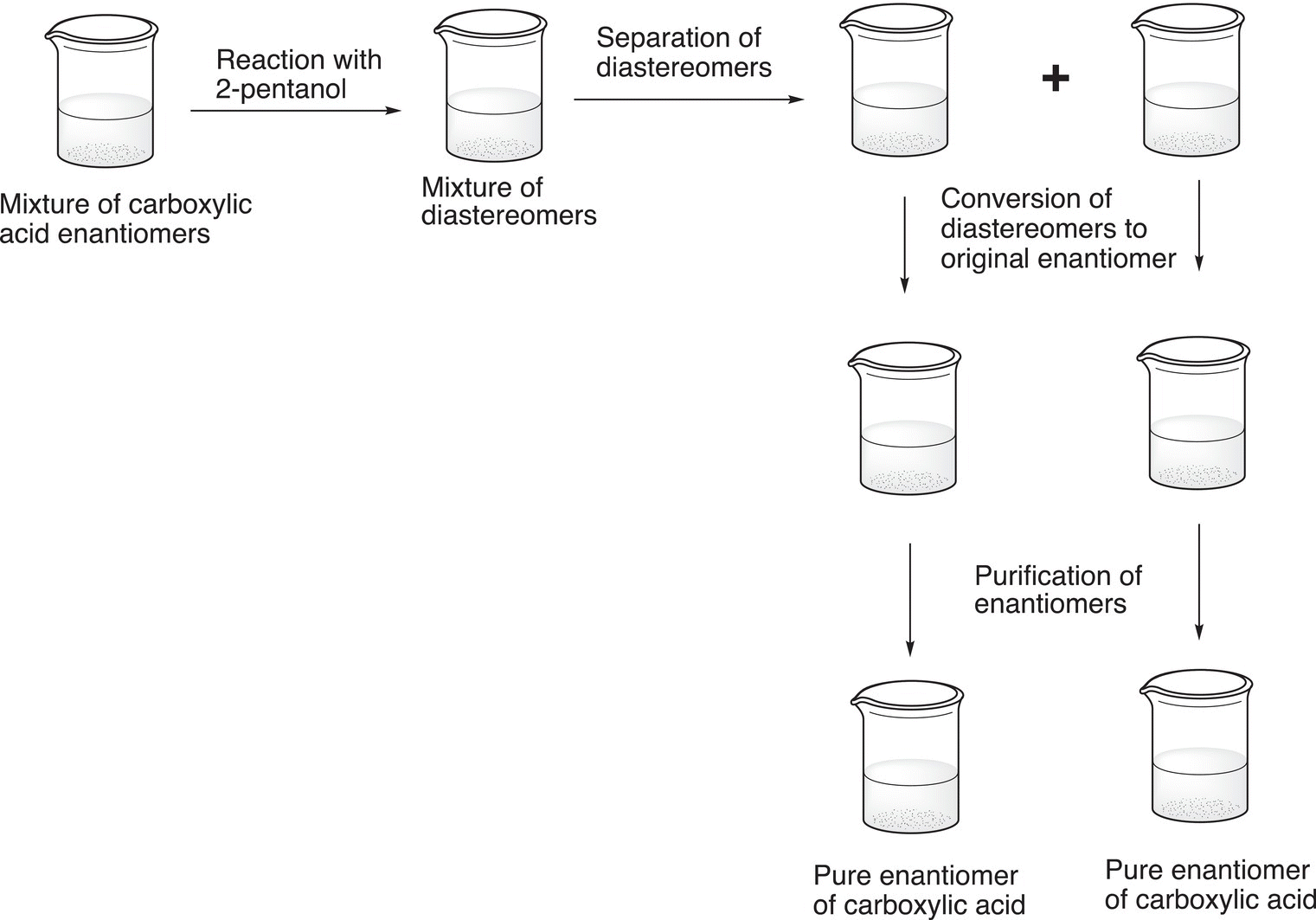
Figure 5.15 Illustration of purification of carboxylic acid by the scheme shown in Figure 5.14.
(5-2)![]()
For the reaction below, a special catalyst was used to achieve a percent enantiomeric excess (%ee) of 76% of one enantiomer over the other.
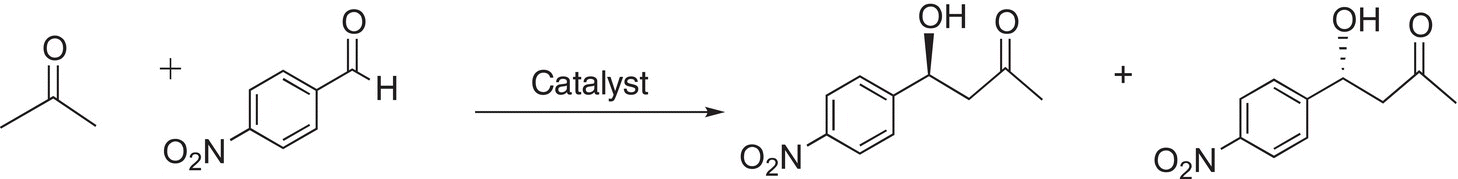
For some reactions, special catalysts can give up to 99%ee, which is extremely important in the pharmaceutical industry since as pointed out in the introduction section, one enantiomer can be poisonous to the body, whereas the other is active. Another example is in the use of Baker’s yeast for the following conversion.
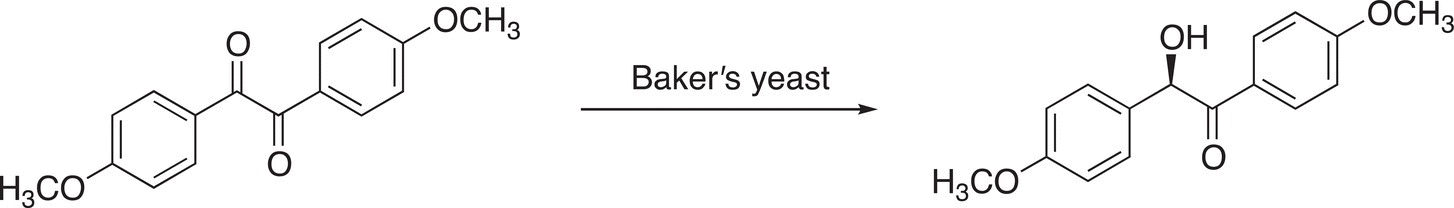
For this reaction, an 84%ee can be obtained.