Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Acid—Base Reactions in Organic Chemistry
7.6 Applications of Acid—Bases Reactions in Organic Chemistry
Some of the strong bases encountered in general chemistry include sodium hydroxide, potassium hydroxide, sodium carbonate, and so on, but in organic chemistry, much stronger bases are required to remove acidic hydrogens of some organic compounds. Based on the information contained in the pKa table, it is possible to predict strong conjugate bases, and it is obvious that one of the strongest organic bases would be the conjugate base of an alkane, since it
is one of the weakest organic acids. Reaction (7-26) shows the conjugate base that results from the deprotonation of methane.
(7-26)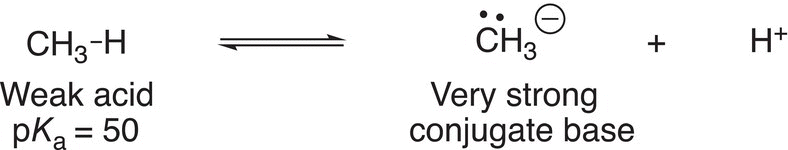
It turns out that there is not a base strong enough to carry out the deprotonation as shown above to generate the conjugate base of an alkane. As a result, the conjugate base has to be synthesized indirectly. Victor Grignard, a French chemist (1871—1935), was one of the first chemists to recognize that compounds of the type shown in Reaction (7-27), could be synthesized by reacting an alkyl halide with magnesium. He received the Nobel prize in chemistry, along with Paul Sabatier, for this discovery.
(7-27)
A close inspection of the product of this reaction, an organomagnesium, shows that the carbon is bonded to magnesium, which is a polar covalent bond in which the bonding electrons reside mostly on the carbon since magnesium is more electropositive. Reagents of this type are essentially the conjugate bases of alkanes, and this procedure can be used to synthesize a wide variety of organomagnesium (or very strong conjugate bases of alkanes). Since Victor Grignard was the first to discover this type of reaction, this type of reaction of an alkyl halide with magnesium is known as the Grignard reaction and the product is the organomagnesium, which is also known as a Grignard reagent. The reaction of a Grignard reagent with phenol is shown in Reaction (7-28).
(7-28)
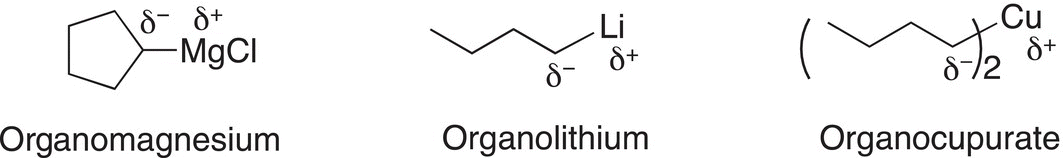
Since this discovery, many different organometallic compounds have been synthesized using different metals. Shown below are some commonly used organometallic reagents, including an example of the Grignard reagent, these are all extremely strong bases.
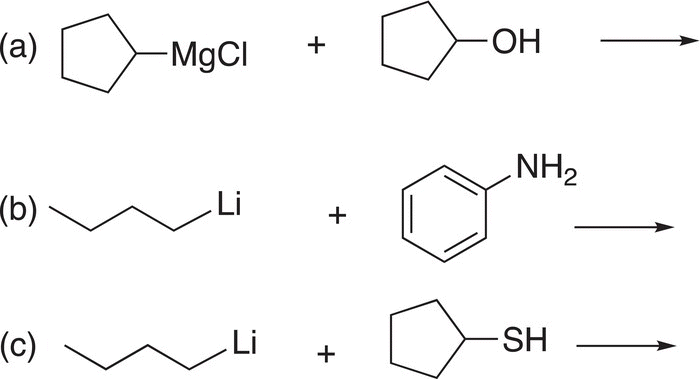
Problem 7.12
Give the products for each of the following acid—base reactions.
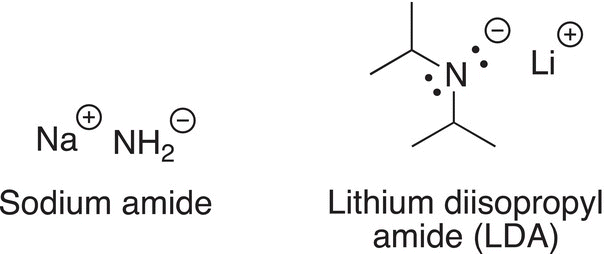
The conjugate base of ammonia is also a very strong base that is commonly used in organic chemistry, and its structure is shown in Reaction (7-29).
(7-29)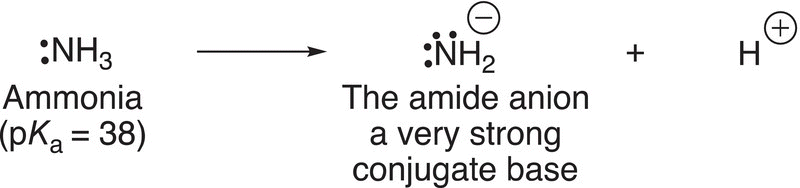
Since the conjugate base is an anion and there is a charge on the nitrogen atom, it is known as a nitrogen base; shown below are two commonly used nitrogen bases in organic chemistry.
Another category of strong bases that are used in organic chemistry is the conjugate bases of hydrogen, the hydride anion, as shown in Reaction (7-30).
(7-30)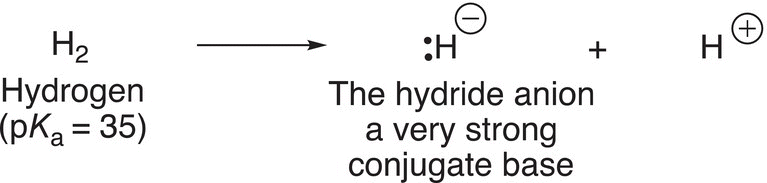
Shown below are examples of hydrogen bases that are routinely used in organic chemistry.
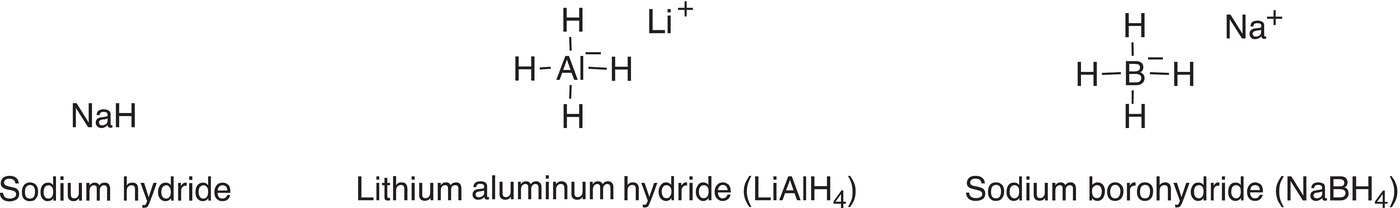
Shown in Reaction (7-31) is an example of a reaction in which LiAlH4 is used as a base.
(7-31)
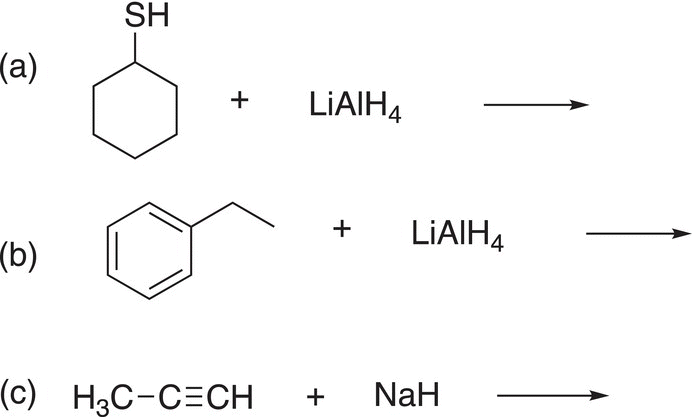
Problem 7.13
Give the products for each of the following acid—base reactions.
Another category of strong bases that are commonly used in organic chemistry are the conjugate bases of alcohols, as shown in Reaction (7-32).
(7-32)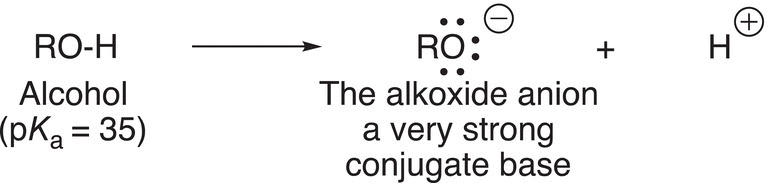
Shown below are some oxygen bases that are routinely used in organic chemistry.
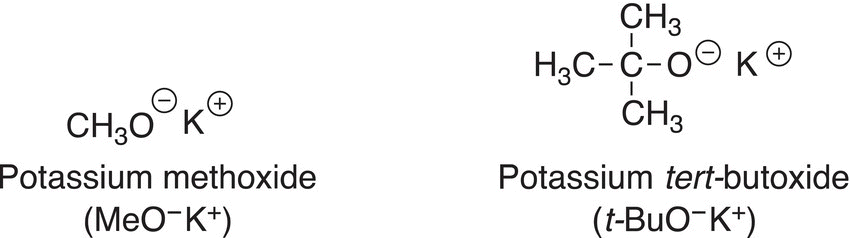
Using the hard—soft description mentioned in the previous chapter, potassium methoxide is relatively small in size, hence a hard base; on the other hand, potassium tert-butoxide is relatively large and classified as a softer base, an example of an acid—base reaction in which an oxygen base is used is shown in Reaction (7-33).
(7-33)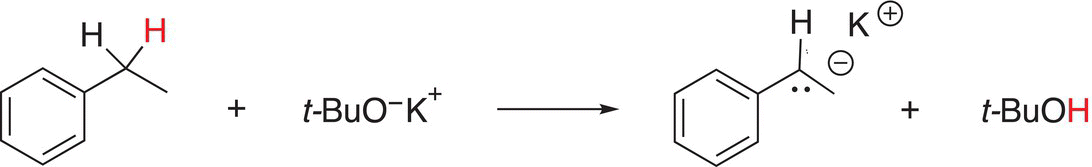
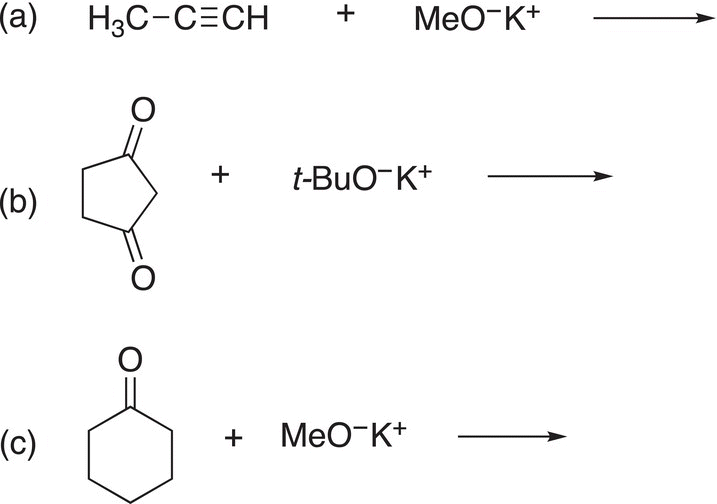
Problem 7.14
Give the products for each of the following acid—base reactions.
Knowledge of pKa values and acid strengths is necessary for choosing appropriate bases for the deprotonation of just about any organic molecule. It is very important that a strong enough base is used for appropriate deprotonation. Extremely strong bases are often very expensive and very reactive. At times, it may be necessary to use a cost-effective weaker base, which can accomplish the same deprotonation task. Curved arrows are typically used to show how the unshared pair of electrons of the base are used to abstract the proton from an organic acid. An example of the curved arrow formulism is shown in Reaction (7-34), in which ammonia abstracts a proton from acetic acid.
(7-34)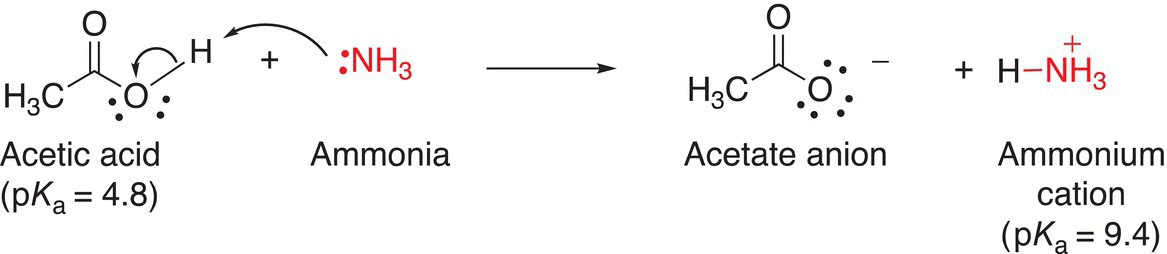
Note that in this notation, a curved arrow with two barbs indicates the movement of two electrons from the ammonia that abstracts the proton from acetic acid. Also, note that the electrons from the ammonia (in the reactant) form a single bond with the proton (H+) that was abstracted to form the ammonium ion in the product, which has a formal charge of positive one (+1). Also, note that since acetic acid has lost a proton (H+), but keeps the bonding electron, it acquires a formal charge of negative one (−1) to form the acetate ion in the product.
Since the double bond of an alkene consists of a pair of π (pi) electrons, alkenes can be considered as Lewis bases and can react with an acid, such as HCl, as shown in Reaction (7-35).
(7-35)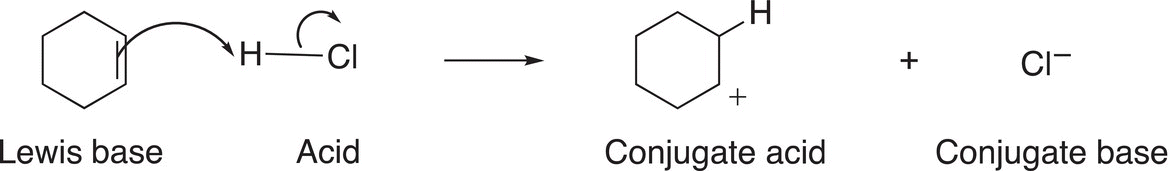
The conjugate acid (also known as a carbocation) that is shown in the reaction of Reaction (7-35) is sp2 hybridized and has an empty p orbital; it can accept a pair of electrons, and hence, a Lewis acid. As a result, carbocations can react with a base in an acid—base reaction as shown in the reaction of Reaction (7-36).
(7-36)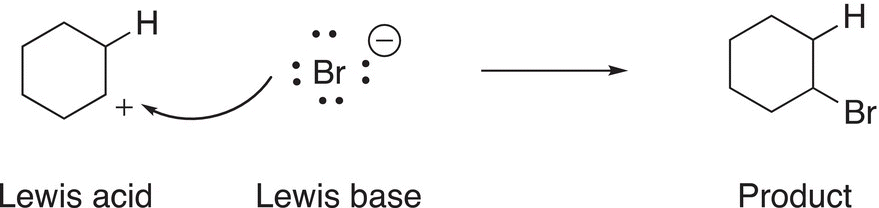
Another example in the use of an appropriate base for an acid—base reaction includes the deprotonation of the α-hydrogen of carbonyl compounds, which is shown in the reaction in Reaction (7-37).
(7-37)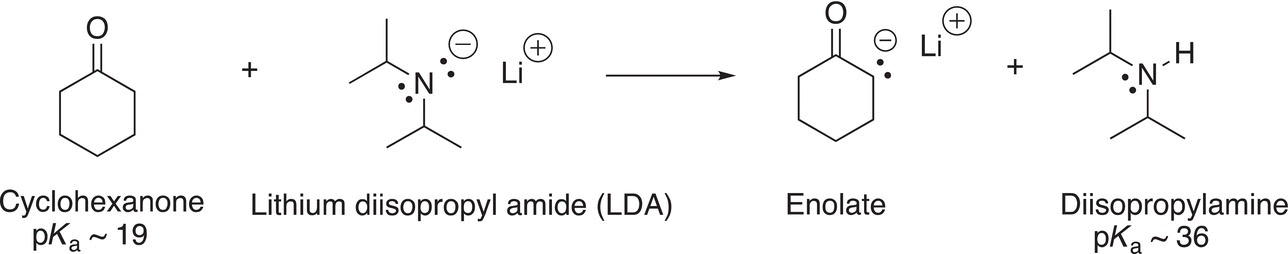
The introduction of a second carbonyl group adjacent to the α-carbon makes the α-hydrogens more acidic, compared to the carbonyl compound given in Reaction (7-37). As a result, a weaker base can be used to remove the proton to form the conjugate base as shown in Reaction (7-38).
(7-38)β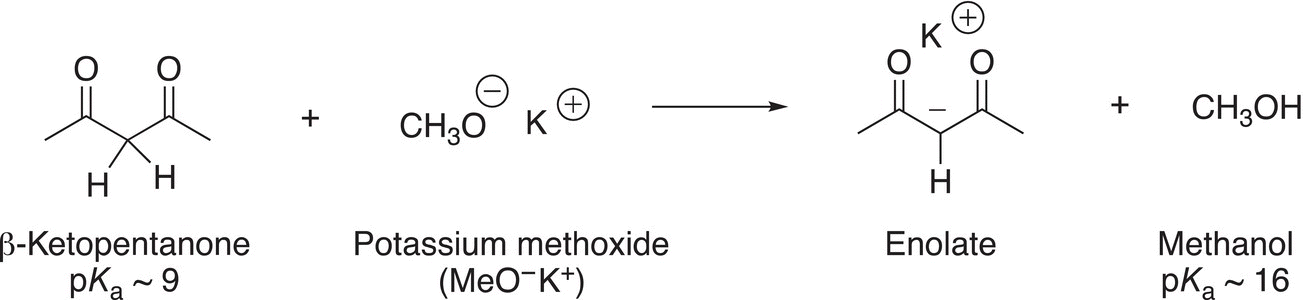
Esters are another category of compounds that contain at least one α-hydrogen and are acidic compounds. With an appropriate base, deprotonation of an α-hydrogen from esters can occur as shown in Reaction (7-39).
(7-39)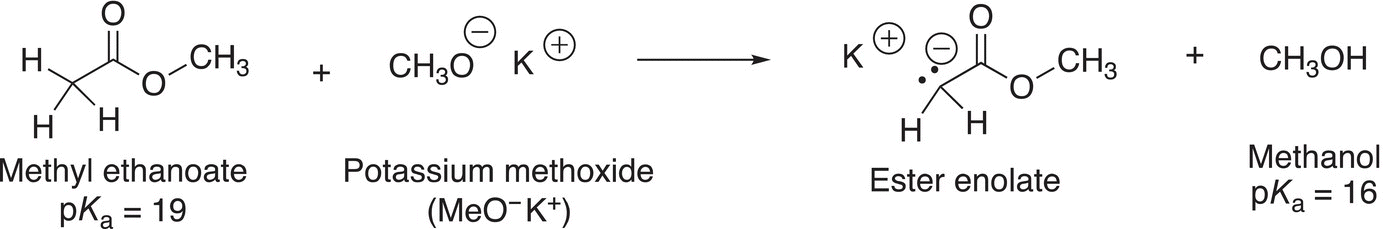
The conjugate base products of the reactions given in the examples above are very important compounds for the synthesis of larger organic molecules, and they will be used in later chapters.