Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Addition Reactions Involving Carbonyls and Nitriles
9.3 Addition of HCN to Carbonyl Compounds
Owing to the polarity of the covalent bond of hydrogen cyanide (HCN), it will add across the carbon—oxygen double bond in a regiospecific manner as shown in Reaction (9-7) to form a new type of compound called a cyanohydrin.
(9-7)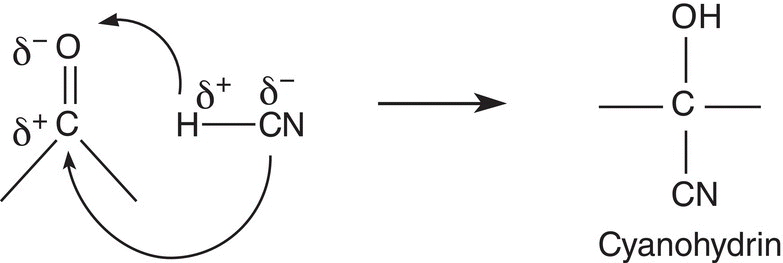
HCN is not very acidic (pKa = 9.2), and it has been shown that reactions of the type as shown in Reaction (9-7) are best carried out in a slightly basic medium and typically catalyzed by KCN or NaCN. In the presence of a carbonyl compound, the CN− will add first to the electrophilic carbon of the carbonyl bond as shown in Reaction (9-8).
(9-8)
In a second step, the basic oxygen abstracts a proton from HCN to give a product called a cyanohydrin and in the process the CN− catalyst is regenerated as shown in Reaction (9-9).
(9-9)
An example of the addition of HCN to benzaldehyde in the presence of NaCN leading to the formation of the cyanohydrin is shown in Reaction (9-10).
(9-10)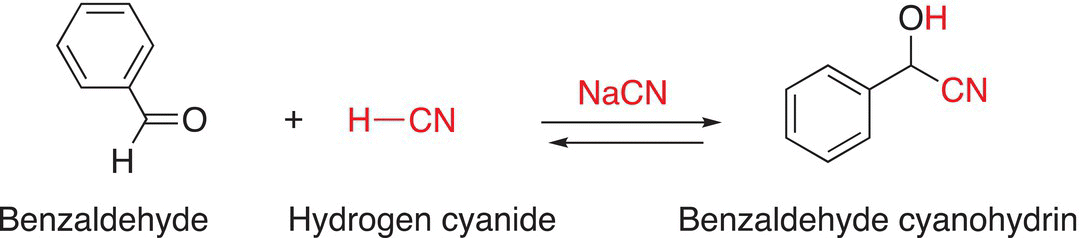
Another example of the addition of HCN to ketone is shown in Reaction (9-11), in which HCN is added to 2-pentanone in the presence of NaCN.
(9-11)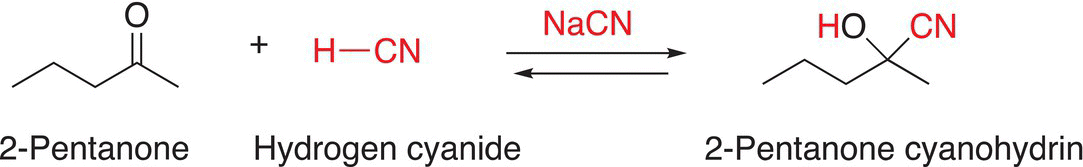
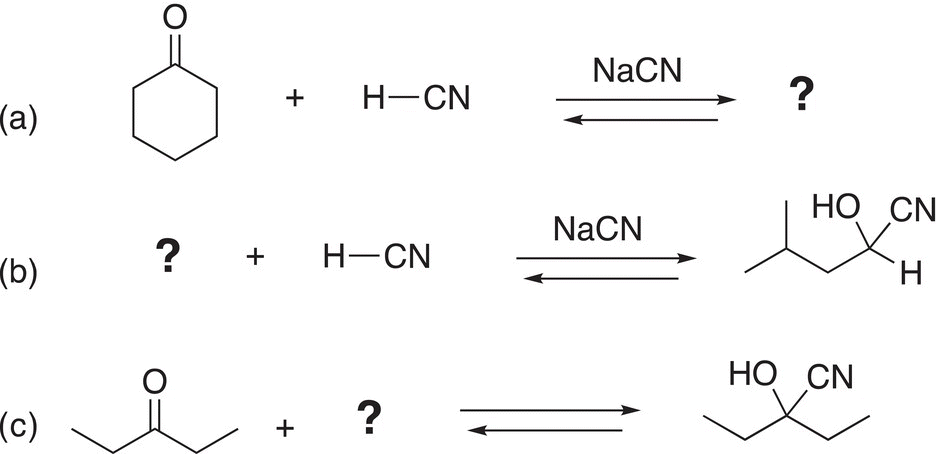
Problem 9.1
Complete the following addition reactions by supplying the major organic product, reactants, or the reaction conditions (appropriate catalyst) to give the products shown.
At this point, it may be a concern about the importance of this type of reaction, and specifically cyanohydrins? These types of reactions can serve to produce intermediates for the synthesis of other important compounds. For the synthesis of α-amino acids, the cyanohydrin can serve as an intermediate as shown in Reaction (9-12) in the synthesis of alanine; the details for the last few reactions will be covered in later chapters.
(9-12)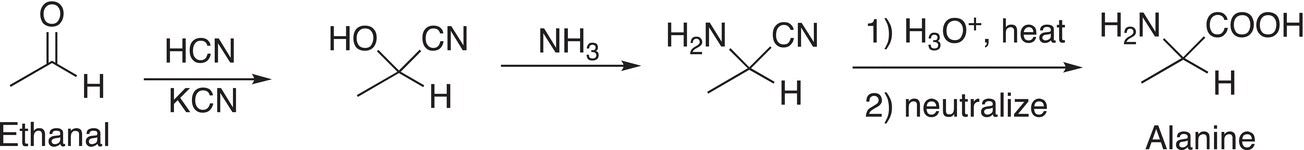
Problem 9.2
Using Reaction (9-12) as a model, show how the cyanohydrin intermediate can be used to synthesize the following amino acids. This problem is designed to get students to start thinking in reverse. That is, you will have to look at the product in the above example and work backward to figure out the starting aldehyde!