Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Reduction Reactions in Organic Chemistry
10.3 Reduction of C=O and C=S Containing Compounds
In this section, the reducing agents discussed in the previous section will be applied to specific type of compounds. As pointed out earlier, the C=O and C=S functionalities have polarized bonds in which the electrons are closer to the oxygen or sulfur compared to the carbon. As a result, the carbon in the C=O and C=S bonds is partially positive, and this region of these molecules is electrophilic and can accept electrons from a reducing agent. In this section, we will utilize the reducing reagents discussed in the previous section to reduce various compounds that have the C=O and C=S functionalities to produce new compounds with different functional groups.
10.3.1 Reduction Using NaBH4 and LiAlH4
The reduction of compounds that have the C=O functionality by LiAlH4 gives alkoxide salts. A condensed mechanism for the reaction is shown in Reaction (10-8).
(10-8)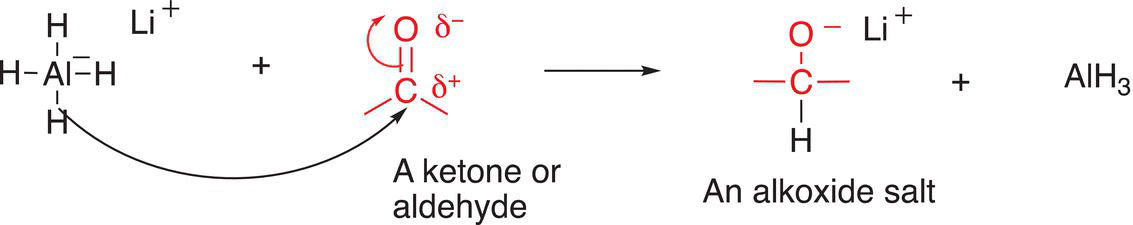
Note that it is the hydride anion (a hydrogen atom with two electrons) that is transferred from the reducing agent, lithium aluminum hydride, to the carbon of the carbonyl functionality (shown in red). In another step, the alkoxide salt, which is strongly basic, can be converted into an organic molecule by the abstraction of a proton from an acid to give an alcohol; shown in Reaction (10-9) is the acid—base reaction of the basic alkoxide salt with dilute HCl to give an alcohol.
(10-9)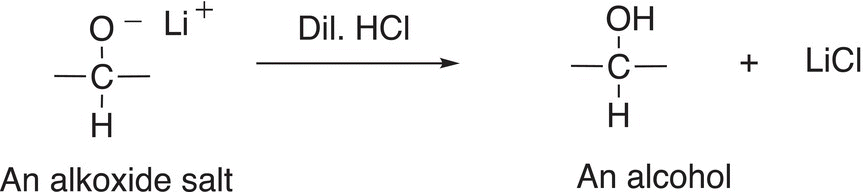
Note that for the two steps of the reaction sequence given in Reactions (10-8) and (10-9), a carbonyl compound is transformed into a different functionality, an alcohol. The sequence of reactions can be summarized in the reaction format shown in Reaction (10-10).
(10-10)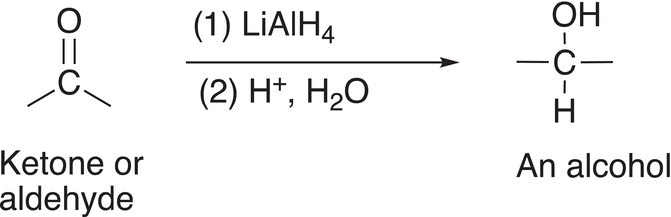
Note that in the second step, that a generic symbol for a dilute inorganic acid (H+) is used; this symbol is typically used to represent one of the general inorganic acids: HCl, H2SO4, or HNO3, and this step is sometimes referred to as a hydrolysis step or acidic workup. Also, note that the inorganic salt is not typically included as a product; typically, just the organic products are shown for organic reactions. A similar reaction can be carried out using NaBH4, and that type of reaction (including the hydrolysis) is shown in Reaction (10-11).
(10-11)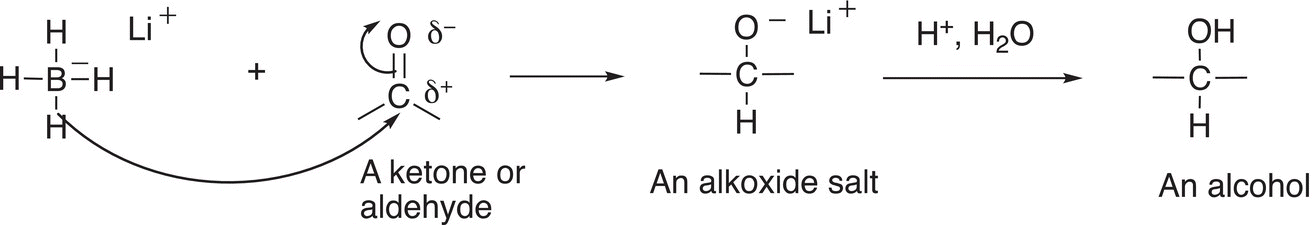
You will readily notice that from these reactions, different types of alcohols result depending on the structure of the starting carbonyl compound. As you saw from Chapter 3, an aldehyde has an alkyl group and a hydrogen bonded to the carbonyl carbon. Whereas, a ketone has two alkyl groups bonded to the carbon of the carbonyl carbon. Formaldehyde is a specific carbonyl compound, which has two hydrogens bonded to the carbonyl carbon.
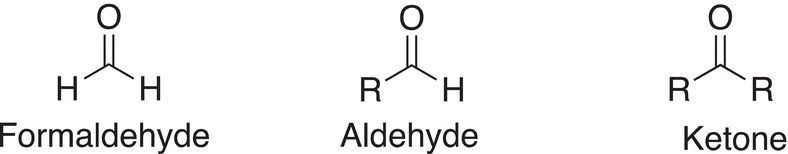
The reduction of formaldehyde with reducing agents that supply the hydride anion (H:−) gives methanol as a product; the reduction of aldehydes gives primary alcohols as the products; and the reduction of ketones gives secondary alcohols as the products. Examples of the reduction of these types of compounds are shown in Reactions.
(10-12)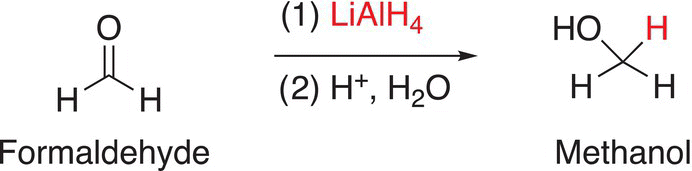
(10-13)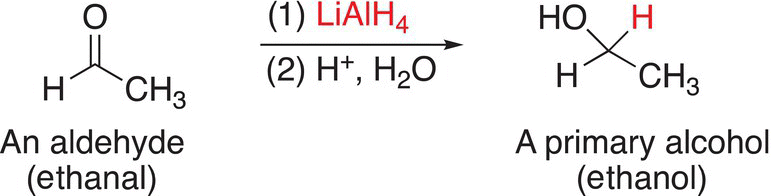
(10-14)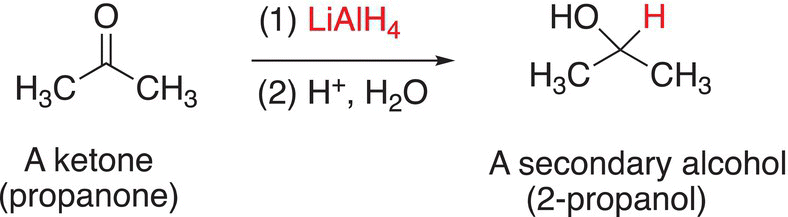
It is not surprising due to the similarity of the structure of C=S functionality to the carbonyl functionality that reactions involving these compounds with a hydride as the reducing agent are very similar and predictable. An example of the reduction of a C=S functionality to produce the thiol functionality is shown in Reaction (10-15).
(10-15)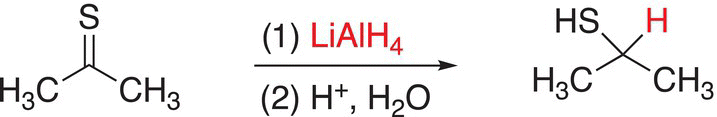
Problem 10.2
i. Give the major organic products of the reduction of each of the following compounds with LiAlH4, followed by hydrolysis.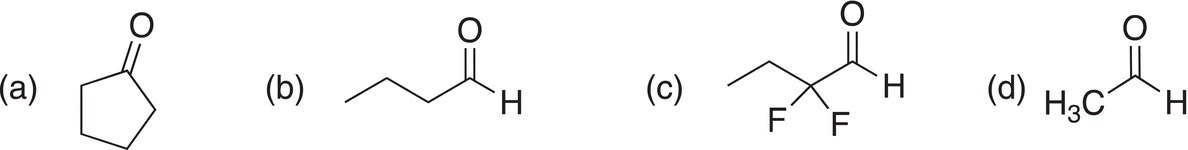
ii. Complete the following reactions by supplying an appropriate reactant or the major organic product.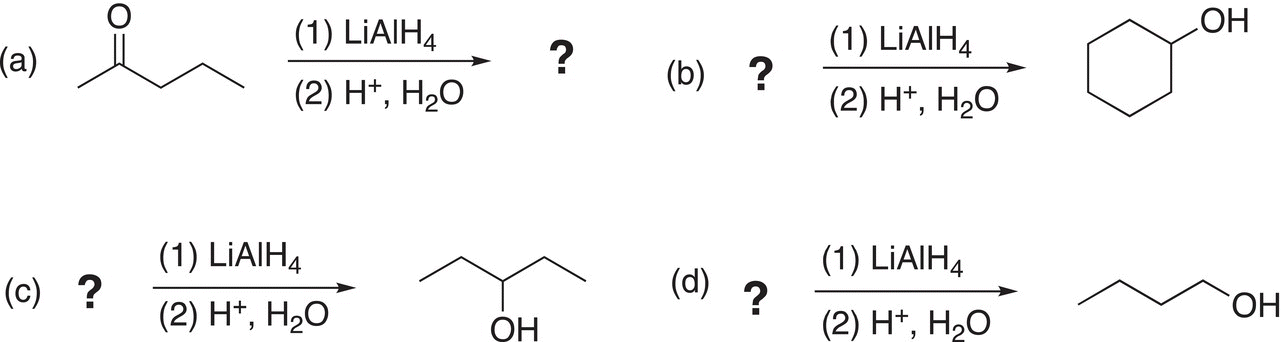
10.3.2 Reduction Using Organometallic Reagents
We learned in Chapter 7 that the conjugate bases of very weak acids are extremely strong Lewis bases; the conjugate bases of alkanes are known as carboanions, and as mentioned earlier, they exist in the form of neutral organometallic compounds. As a result, these compounds are also extremely good reducing agents and react with the electrophilic carbons of C=O and C=S bonds, similar to that of the reaction of the hydride anion with these types of compounds, but the type of organic products produced from these reactions are different from that of the reaction with metal hydrides. For these reactions, a new carbon—carbon bond is formed, instead of a C─H bond, as observed from the reaction involving metal hydrides. One of the most popular organometallic compounds is the Grignard reagent (RMgX), where X represents a halogen, usually chlorine or bromine. The mechanism for the reaction of Grignard reagents with the C=O functionality is very similar to that involving the reaction of the hydride ion, in this case, the nucleophilic carbon of the alkyl group adds to the carbonyl carbon and forms a new carbon—carbon bond, as shown in the generic mechanism of Reaction (10-16).
(10-16)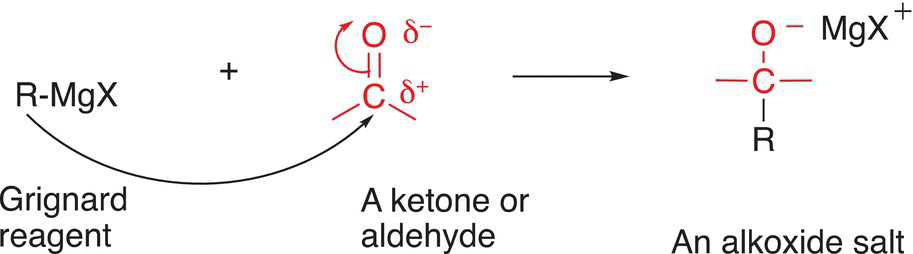
As pointed out in the previous section, a second step that involves an acid—base reaction (hydrolysis) of the alkoxide salt will convert the basic salt to an alcohol, as shown in Reaction (10-17).
(10-17)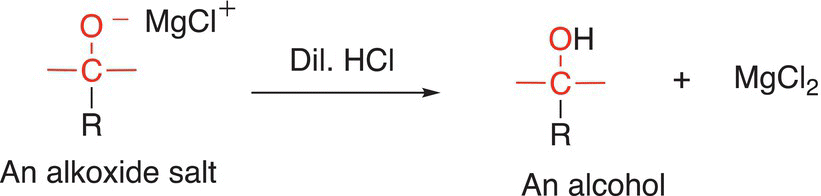
You will notice that this sequence of reactions transforms a carbonyl functionality to an alcohol, with a new carbon—carbon bond in the product. The reaction of formaldehyde, which has two hydrogens bonded to the carbonyl functionality, with a Grignard reagent gives a primary alcohol after hydrolysis of the alkoxide salt. The reaction of a Grignard reagent with aldehydes gives secondary alcohols after hydrolysis of the alkoxide salts, and the reaction of a Grignard reagent with ketones gives tertiary alcohols after hydrolysis of the alkoxide salts as shown in Reactions.
(10-18)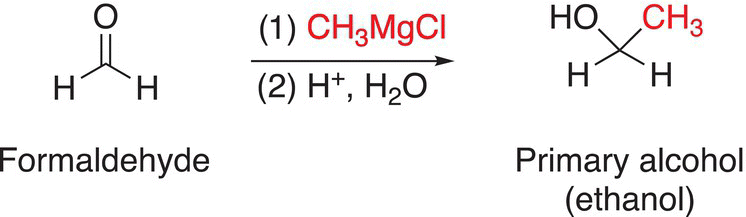
(10-19)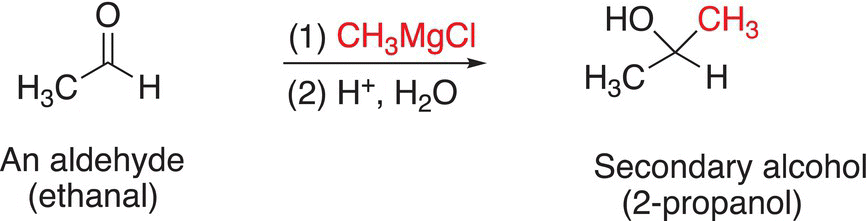
(10-20)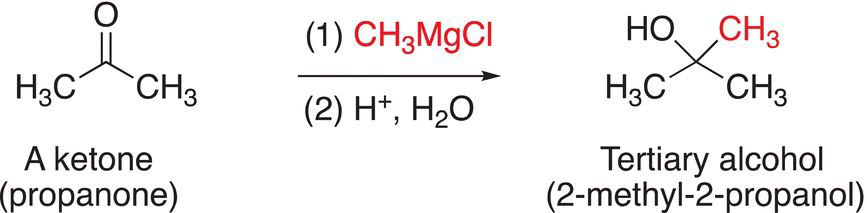
An example of the reduction of a C=S functionality to produce the thiol functionality using methyl Grignard is shown in Reaction (10-21); in this case, the product is not an alcohol, but a thiol.
(10-21)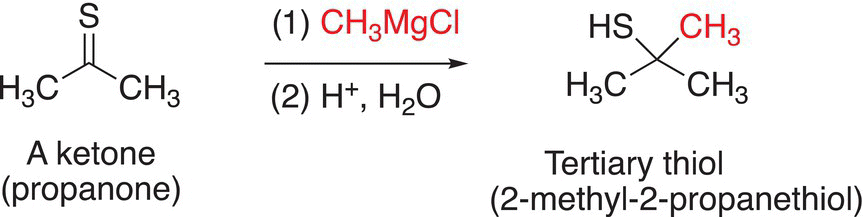
Problem 10.3
i. Show how you could use a compound that contains a C═O or C═S functionality and an appropriate Grignard reagent to synthesize the molecules shown below.
ii. Tertiary alcohols can be synthesized by the reaction of a Grignard reagent (RMgX) with an appropriate ketone. Outline three different syntheses of 3-methyl-3-hexanol, but utilizing different Grignard reagents and ketones in each of your synthesis.
An important reduction reaction using organometallic reagents is its reaction with carbon dioxide, as shown in Reaction (10-22).
(10-22)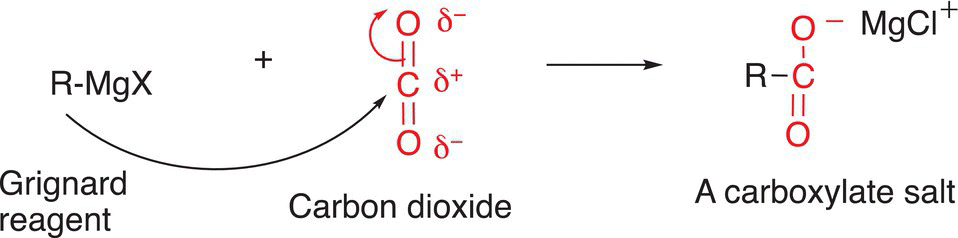
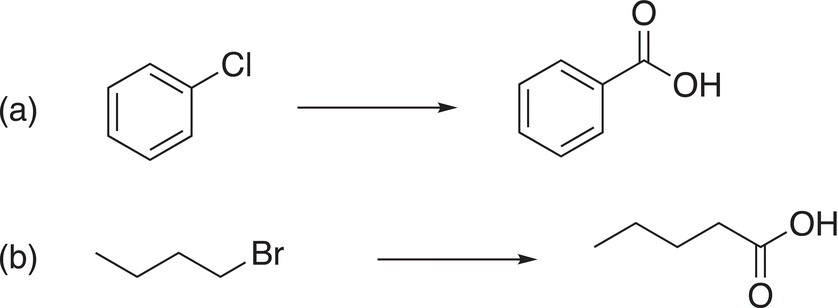
As we have seen, these types of basic salts can be converted into a nonionic organic molecule in the presence of a weak acid, as shown in the reaction given in Reaction (10-23). In this case, the final organic products are carboxylic acids.
(10-23)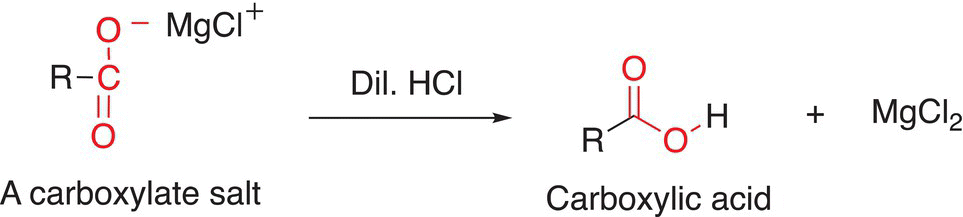
Note that this transformation using a Grignard reagent to make carboxylic acid increases the number of carbons in the carboxylic acid product.
Problem 10.4
Show how to carry out the following transformations, your synthesis should involve a Grignard reagent and carbon dioxide.
10.3.3 Reduction Using Acetylides
We have mentioned earlier in Chapter 7 that terminal alkynes are relatively acidic, and the acidic hydrogen can be deprotonated with an appropriate base. The acetylide anion that results is not only a conjugate Lewis base but is also a potential reducing agent. As you can imagine, it is not as strong a reducing agent, compared to the reducing agents covered earlier since the carbon of acetylides are more electronegative than that of alkyl carbons, but nonetheless, the acetylide anion can give its pair of nonbonding electrons to an electrophile to form a new carbon—carbon bond. Examples are shown in Reactions and note that the products of these reactions are not only alcohols but also difunctional containing an alcohol and an alkyne functionality.
(10-24)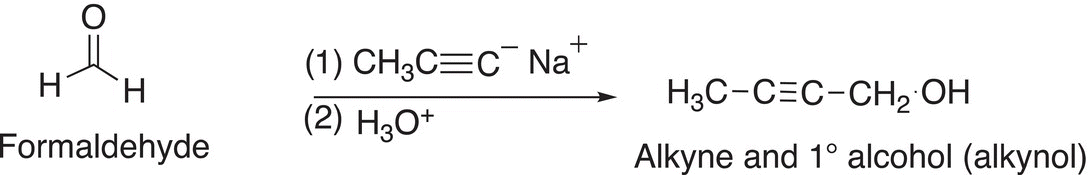
(10-25)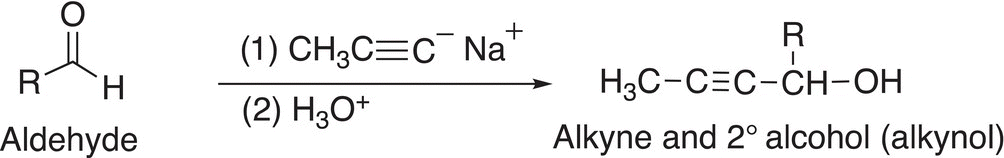
(10-26)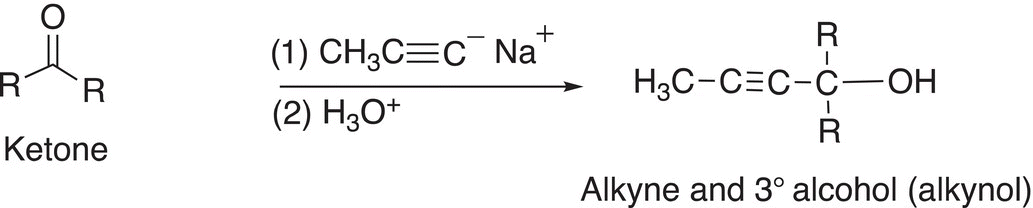
At this point, you are beginning to realize that with much creativity, you can devise the synthesis of a wide variety of different compounds by using various reaction types and carefully selecting specific reactants and specific reducing agents. The result is that new molecules with different functional groups are produced.
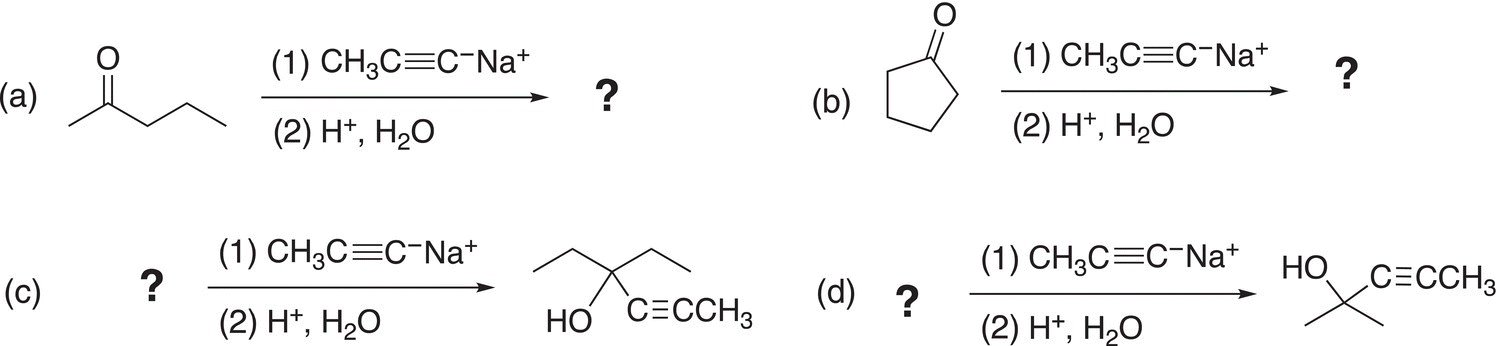
Problem 10.5
Complete the following reactions by supplying an appropriate reactant or the major organic product.
10.3.4 Reduction Using Metals
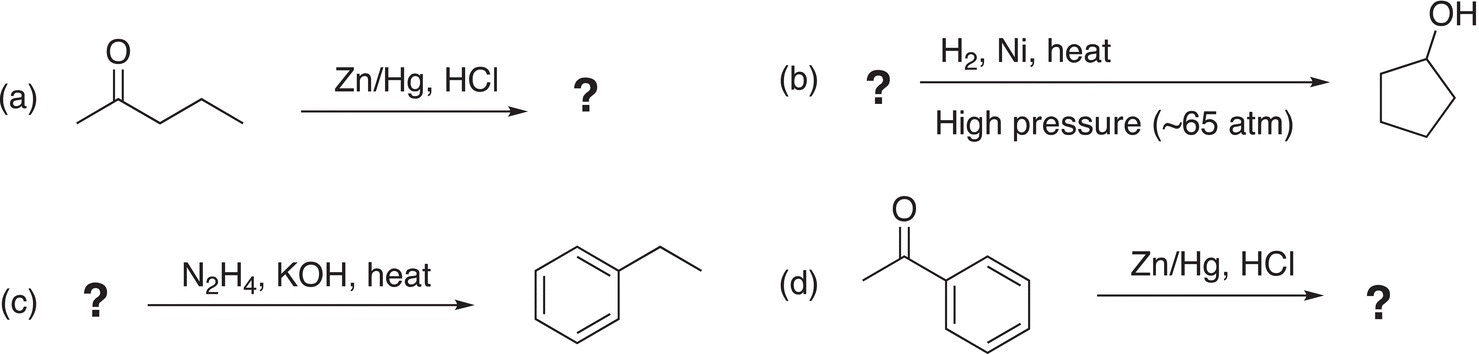
This type of reduction is very important since a carbonyl functional group can be reduced to a methylene unit using transition metals, such as zinc. One of these types of reactions is shown in Reaction (10-27) and is often referred to as the Clemmensen reduction.
(10-27)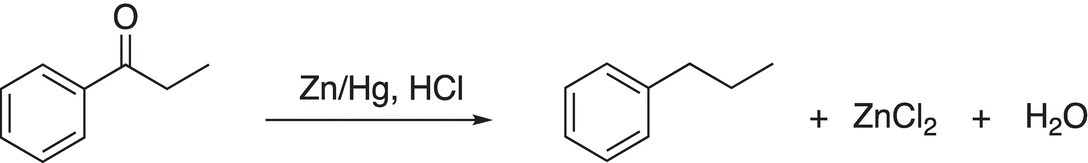
This reaction is named after a Danish chemist, Erik Christian Clemmensen (1876—1941). In this reaction, an alloy of zinc/mercury amalgam is used as the reducing agent. In the first step of the mechanism, the carbonyl oxygen is protonated in the acidic medium, which makes the carbonyl carbon even more electrophilic. The next step involves the delivery of an electron pair from zinc to the protonated carbonyl compound, and in another step of the reaction mechanism, the chloride anion reacts with the reduced intermediate as shown in Reaction (10-28).
(10-28)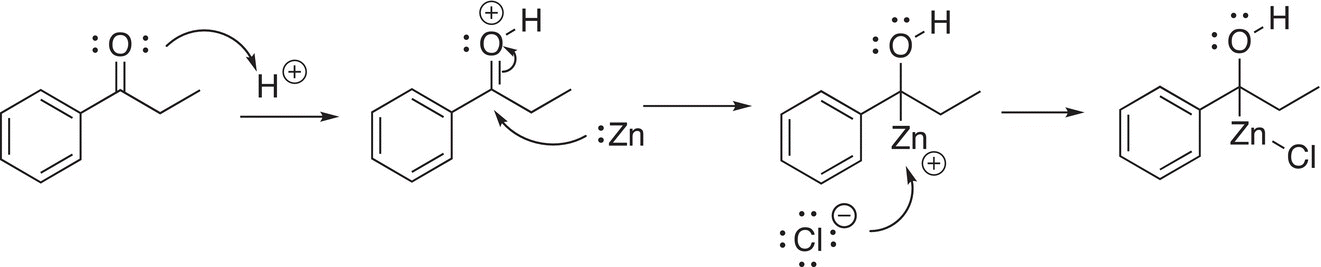
In the next step of the reaction mechanism, water is lost in the acidic medium to form a cationic intermediate, to which another mole of zinc delivers another pair of electrons as shown in Reaction (10-29).
(10-29)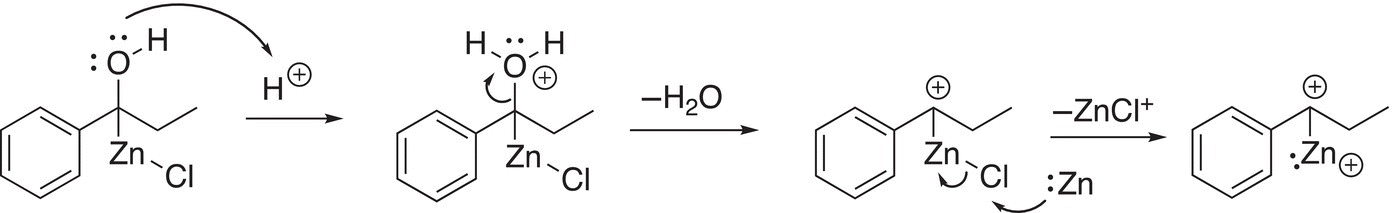
In the next step, the electrons are delivered to the carbocation, converting it into a carboanion shown in Reaction (10-30).
(10-30)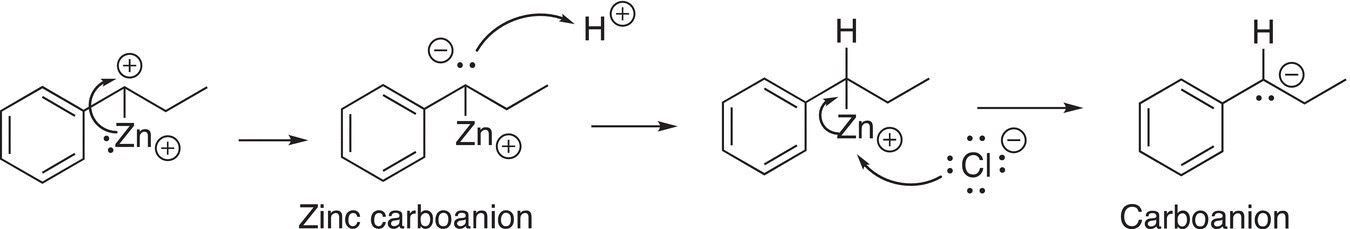
In the last step of the reaction mechanism, the carboanion reacts with the proton of the acidic medium to give the reduced product as shown in Reaction (10-31).
(10-31)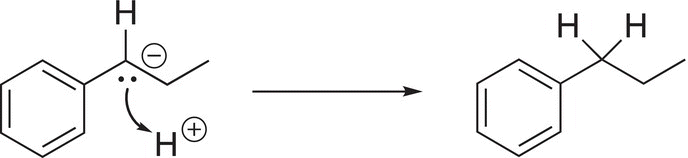
10.3.5 Reduction Using Hydrogen with a Catalyst
In general chemistry, we learned that reduction is typically accompanied by the gain of hydrogen. Hydrogen, in the presence of a catalyst, is routinely used in organic chemistry to accomplish reduction of carbon—carbon double and triple bonds. The reduction of carbon—oxygen double bonds and carbon—nitrogen double and triple bonds can be accomplished by hydrogenation, but more severe conditions, such as high temperatures and pressures in the presence of an appropriate catalyst, are required. The reduction of a molecule that has a carbon—oxygen double bond using hydrogen in the presence of a catalyst is shown in Reaction (10-32).
(10-32)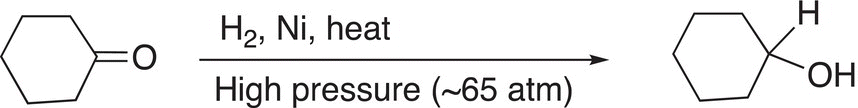
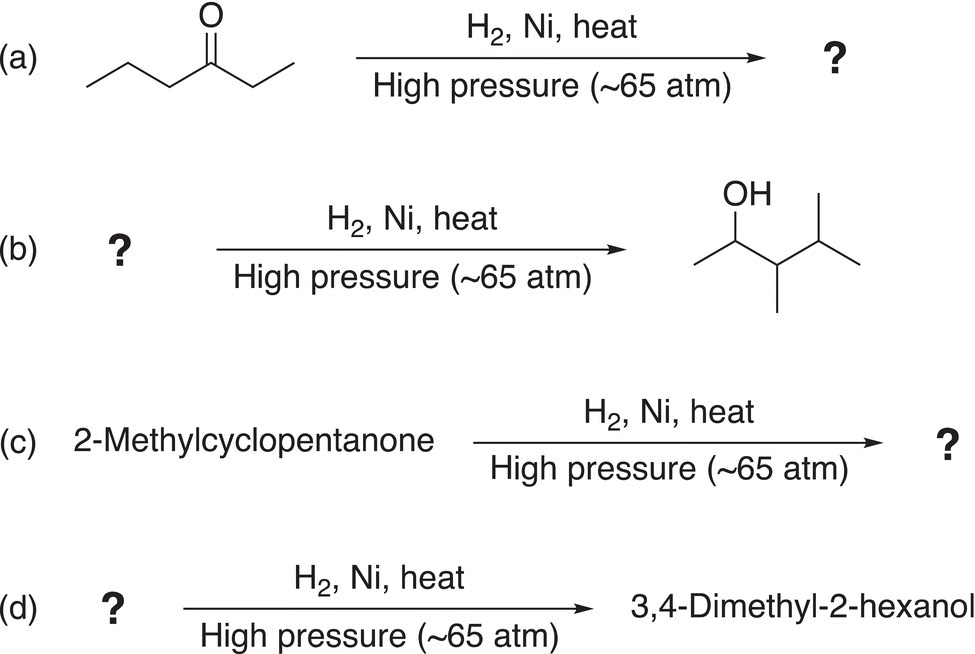
Problem 10.6
Give the reactant or the organic product to complete the reactions shown below.
10.3.6 The Wolff Kishner Reduction
The addition of hydrazine to a carbonyl functionality gives a product that we have seen in the previous chapter, called a hydrazone (Reaction 10-33).
(10-33)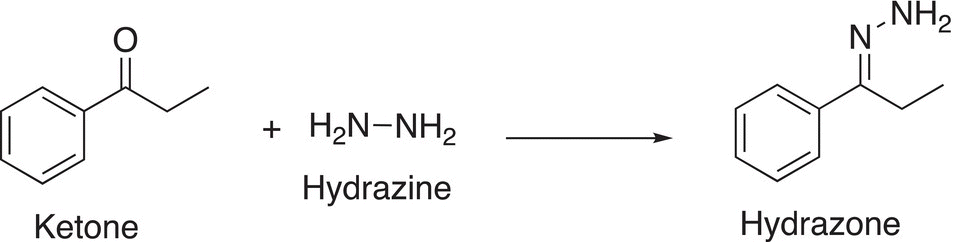
In the presence of a strong base, such as KOH, the hydrazone is reduced and in the process liberates nitrogen gas as shown in Reaction (10-34).
(10-34)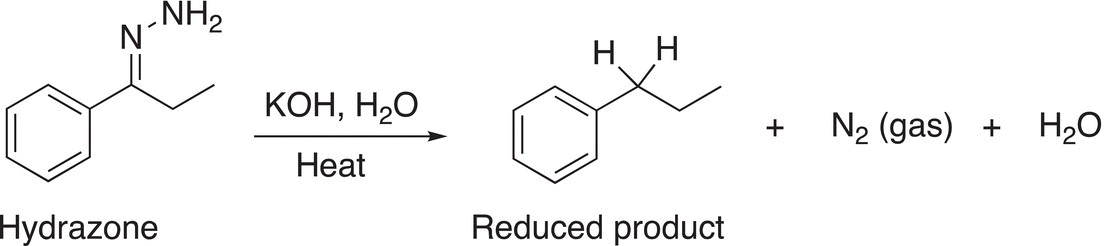
A driving force for this reaction to proceed to the right is the liberation of nitrogen gas (an increase in entropy). The mechanism for this reaction involves the abstraction of a proton bonded to the nitrogen as shown below to generate a carboanion, which abstracts a proton from the solvent, water as shown in Reaction (10-35).
(10-35)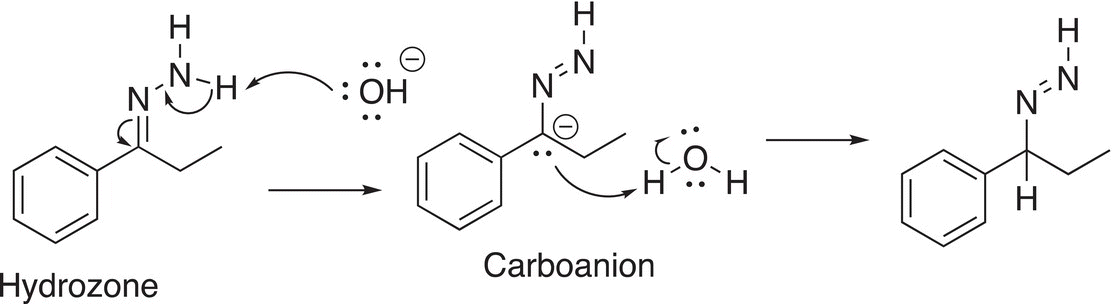
In the next step of the mechanism, another mole of the hydroxide anion abstracts another proton from the hydrogen bonded to the nitrogen, releasing nitrogen gas and another carboanion, which abstracts a proton from the solvent, water to give the reduced product as shown in Reaction (10-36).
(10-36)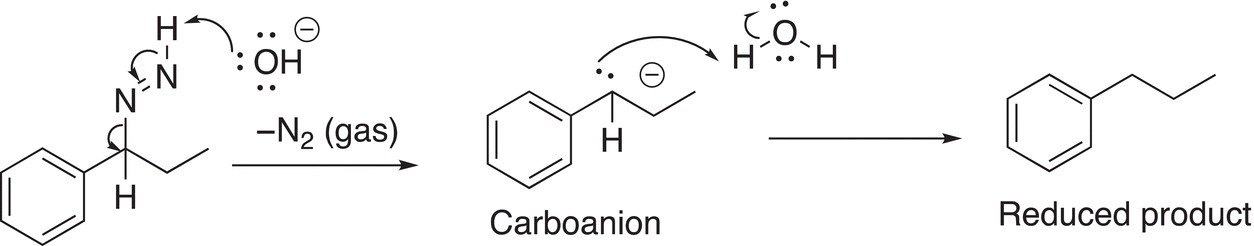
This type of reaction was discovered in the early 1900s by Nikolai Kishner and Ludwig Wolff and now known as the Wolff-Kishner reaction. For the Wolff-Kishner reaction, note that this reduction takes place in a very basic medium, compared to the Clemmensen reduction, which takes place is an acidic medium. Reaction (10-35) gives an example of the Wolff-Kishner reduction reaction.
(10-37)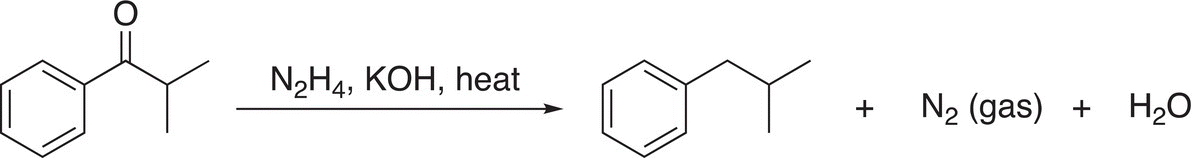
At the end of this chapter, we will demonstrate the need for carrying out reduction under different conditions, such as acidic versus basic condition, and an appropriate reduction must be selected in order to accomplish the synthesis of specific target molecules.
Problem 10.7
Complete the following reactions by supplying an appropriate reactant or the major organic product.