Cracking the SAT Chemistry Subject Test
Part IV
Practice SAT Chemistry Subject
Tests and Answers and Explanations
Chapter 20
Practice SAT Chemistry Subject Test 3: Answers and Explanations
PART A
1 E The composition of Earth's atmosphere is approximately 78% N2, 20% O2, 1% Ar, 0.5% H2O, 0.4% CO2, and 0.1% other trace gases.
2 A Allotropes are different forms or molecular arrangements of the same element. Carbon has three common allotrophic forms at standard conditions (25°C and 1 atm): amorphous carbon (charcoal), graphite, and diamond. Graphite is unique among nonmetals in that it conducts electricity.
3 B Paramagnetic means that the atom or molecule has at least one unpaired electron. Since it is impossible to completely pair up an odd number of electrons, atomic nitrogen must always be paramagnetic because it has an odd number of electrons (7).
4 B Two elements that are essential to plant growth but are depleted in most soils are nitrogen and phosphorous. Phosphorous is not given as a choice, but nitrogen is. Plants cannot utilize atmospheric nitrogen gas because the strong triple bond in N2 makes it virtually inert to biological processing.
5 C Again, allotropes are different forms or molecular arrangements of the pure element. Oxygen has two allotropic forms at standard conditions: molecular oxygen, O2, and ozone, O3. Ozone (and to a lesser extent, molecular oxygen) is the primary absorber of UV light in Earth's atmosphere.
6 A This is the definition of chemical pH indicators.
7 E Supersaturated solutions are solutions that have an amount of dissolved solute (the solute can be a solid, liquid, or gas) greater than they should at that particular temperature and pressure. Therefore, these solutions are not in equilibrium with their environment. For example, a glass of soda that's just been poured is supersaturated with CO2. The soda tries to reach equilibrium with its environment by ejecting excess CO2 as bubbles of CO2. If left alone for a while, the soda will eventually release all of the excess CO2 and stop forming bubbles because it's reached equilibrium—;at that point we say the soda has gone flat.
8 D Biologists use the terms hypotonic, isotonic, and hypertonic to contrast the osmotic pressure of a cell's cytoplasm with that of the surrounding extracellular solution.
9 B One of the most important intrinsic properties of a buffer is that the addition or removal of water in no way alters the pH of a buffer. In contrast, diluting any of the other solutions is likely to alter their pH—;i.e., [H3O+].
10 D A catalyst decreases the activation energy, or energy barrier, that must be overcome for reactants to become products. In this way, catalysts increase the rate of chemical reactions.
11 C Enthalpy is the fancy word for heat of reaction; it is abbreviated with an “H.” The abbreviations for the other properties listed are standard voltaic potential: E°, entropy: S, reaction rate, and Gibbs free energy: G.
12 B Entropy, S, is the measure of the amount of disorder in a molecular system. When a gas condenses into a liquid, the molecules become more organized, so the entropy of the system decreases.
13 A It may be tempting to choose (E), but remember that by definition, the change in Gibbs free energy, ∆G, must be negative for a reaction to be spontaneous. Standard voltaic potential, E°, is related to ∆G° by the equation
∆G° = –nFE°
where n is the number of transferred electrons and F is Faraday's constant. Since n and F are always positive numbers, you can see that for a spontaneous reaction—;where ∆G° is negative—;E° will always be positive.
14 B By definition, entropy for a pure element in crystalline form at absolute zero (0 K) is zero.
15 C The atoms of noble gas elements have filled valence shells and, therefore, are extremely unreactive—;more so than any other family.
16 D To form a negative ion, an atom needs to acquire electrons. This sounds like a nonmetal, not a metal. Eliminate (A), (B), and (E). Noble gases are essentially inert, so that leaves the halogens. Halogens need 1 valence electron to complete their valence shell and so will readily gain an electron and form an anion.
17 E When the test writers start talking about the “d” subshell, think “transition metals.”
18 D Did you remember this statement: Have No Fear Of Ice Cold Bears? If so, you'd realize that some of the most common diatomic molecules are halogens—;F2, Cl2, Br2, and I2.
19 A Ionization energy is needed to remove an electron from an atom. Which kind of elements tend to give up electrons? Metals, of course. Of the metals, alkali metals, having only 1 valence electron per atom, will lose an electron most easily because this allows an alkali metal atom to assume a stable noble gas electron configuration.
20 B Given that the strong acids are HCl, HBr, HI, HNO3, H2SO4, and HClO4 and the strong bases are LiOH, NaOH, KOH, RbOH, and CsOH, the only compound that could result from a neutralization between a strong acid and strong base is KI.
21 C Compounds composed of only nonmetal elements tend to form covalent bonds, while compounds composed of metals and nonmetals tend to form ionic bonds. Therefore, N2 and CCl4 are expected to be covalent compounds. However, N2 is a gas at standard conditions; only CCl4 is a liquid.
22 E Carbonates, CO32–, and bicarbonates, HCO3−, form CO2 gas when mixed with acid. That's why baking soda (NaHCO3) fizzes when added to vinegar (acetic acid).
23 D Precipitation reactions most often involve single or double displacement reactions between two ionic compounds. Therefore, compounds (A) and (C) are eliminated because they are not ionic compounds. Recall the basic solubility rules for ionic compounds.
• All group 1 and ammonium salts are soluble.
• All nitrate and perchlorate salts are soluble.
• All silver, lead, and mercury salts are insoluble (except for their nitrates and perchlorates).
Therefore, mixing AgNO3 with NaCl should produce the insoluble compound AgCl.
24 B There are only two purple solids commonly found in a general chemistry lab: iodine, I2, and potassium permanganate, KMnO4. Considering that element Mn is not present in any of the answer choices, but I is (in KI), it is likely the purple solid formed here is iodine. In fact, the iodide ion, I−, is easily oxidized to form I2.
25 B The primary source of solar energy is energy released by the nuclear fusion of hydrogen at high pressure and temperature to form helium. 41H → 4He + 2 electrons + lots of energy.
26 A The only nuclear process that does not change the number of protons and neutrons of a nucleus is gamma decay. Gamma decay involves stabilization of a nucleus by loss of energy in the form of a gamma ray photon.
27 C Although nuclear fusion of hydrogen is the chief source of helium in the universe, most of the helium originally caught up in Earth as it formed 4.5 billion years ago has escaped into space because Earth's gravity is too weak to retain helium atoms once they find their way into the atmosphere. However, new helium atoms are constantly being formed as a product of the alpha decay of heavy elements such as uranium and thorium present in Earth's crust. Therefore, oddly enough, people in the helium business actually mine for pockets of helium trapped in underground caverns and fissures; they don't extract it from the air.
28 C Given the nuclear reaction
![]()
When conserving mass and charge, the missing particle must be ![]() He. Therefore, the nuclear process responsible for this transmutation is alpha decay.
He. Therefore, the nuclear process responsible for this transmutation is alpha decay.
29 D A pH of 13 indicates a basic solution; therefore, there must be a base in solution in the first place. NH4OH and KOH are bases. However, a pH of 13 means that the pOH is 1, i.e., [OH−] = 0.1 M. For the [OH−] to be the same as the base, that base must completely dissociate and be strong. KOH is the only strong base given.
30 A This is a question about the colligative property freezing point depression. Remember that for all colligative properties, the greater the number of dissolved particles, the greater the effect. Therefore, this question is really asking which solution has the greatest number of dissolved particles. Since all of them have the same molar concentration, this is really just a contest of which compound breaks up into the most individual particles.
MgCl2 → Mg2+ + Cl− + Cl− (3 particles)
HClO4 → H+ + ClO4− (2 particles)
NH4OH → NH4+ + OH− (2 particles)
KOH → K+ + OH− (2 particles)
LiNO3 → Li+ + NO3− (2 particles)
Therefore, the winner is MgCl2.
31 A This is a question about the colligative property boiling point elevation. As in question 30, recall that for all colligative properties, the greater the number of dissolved particles, the greater the effect. Therefore, this question is really asking which solution has the greatest number of dissolved particles. Since all of them have the same molar concentration, this too is a contest of which compound breaks up into the most individual particles.
MgCl2 → Mg2+ + Cl− + Cl− (3 particles)
HClO4 → H+ + ClO4− (2 particles)
NH4OH → NH4+ + OH− (2 particles)
KOH → K+ + OH− (2 particles)
LiNO3 → Li+ + NO3− (2 particles)
Again, the winner is MgCl2.
32 E Certain metal ions produce characteristic colors when ionized in a flame—;that's how fireworks are made to have different colors. Here are the most common ions, and the color they produce.
|
Red: |
Lithium, strontium |
|
Orange: |
Calcium |
|
Yellow: |
Sodium |
|
Green: |
Barium, copper |
|
Violet: |
Potassium |
Therefore, lithium ions, Li, will produce a red flame—;choice (E).
PART B
101 T, T, CE Divide and conquer. Both statements are true. Nearly all colored compounds fall into two categories: 1) those that are colored because they are organic molecules that have conjugation, and 2) those that are colored because they have transition metal atoms with partially filledd subshells. That's why sodium oxide is colorless, but iron(II) oxide is orange.
102 T, F Divide and conquer. The first statement is true. It is a fundamental law of chemical kinetics that all chemical processes slow down at lower temperatures—;that's why refrigerating food retards the growth of microbes. At lower temperatures, reactant molecules have less kinetic energy to use to overcome the energy barrier for the formation of products, called the activation energy. The second statement is false. The only way to lower activation energy is to add a catalyst.
103 F, T Divide and conquer. Exothermic reactions release heat energy (notice exo– looks like exit), endothermic reactions absorb energy (notice endo– looks like enter), so the first statement is false. The second statement is true and is an important law in chemistry.
104 F, T Divide and conquer. The first statement is false. The solubility of a gas in a liquid is very sensitive to pressure, such that the solubility of gases in liquids increases with increasing pressure. That's why when we release the pressure trapped in a bottle of soda by opening it, a sudden surge of carbon dioxide bubbles races to get out of the container. The second statement is true but has no relevance to the solubility of gases.
105 T, T, CE Divide and conquer. Exactly true. All ionic compounds have relatively high melting points (all are solids at room temperature) because ionic forces between ions are very strong. In the case of MgO, the +2 and –2 charges on Mg and O, respectively, result in very strong intermolecular forces. Not surprisingly, MgO has a melting point—;it's about 2,000°C.
106 T, T, CE Divide and conquer. According to the Aufbau principle, we completely fill subshells before moving up to the next higher one. However, completely half-filled and filled d subshells bestow extra stabilization to an atom. Therefore, Cr and Cu actually violate the Aufbau principle and promote a 4s electron to become [Ar] 4s13d 5 and [Ar] 4s13d 10, respectively. Remember this important exception.
107 T, T, CE Divide and conquer. Isotopes are atoms of the same element that have differing numbers of neutrons. They have nearly identical chemical behavior because the number of protons and electrons in an atom (two quantities that are identical between isotopes) govern an atom's chemical properties.
108 F, F Divide and conquer. Both statements are false. For any electrochemical or electrolytic cell, oxidation occurs at the anode and reduction occurs at the cathode. (Remember: AN OX and RED CAT.) Therefore, both statements are false.
109 T, T, CE Divide and conquer. No matter how complicated acid/base chemistry can appear, never forget that adding acid to any solution, buffered or not, always lowers the pH; adding base to any solution always raises the pH. A buffer does not prevent the pH from changing in these cases; it simply lessens by how much the pH changes.
110 T, F Divide and conquer. The first statement is true. Remember the colligative properties: Adding any solute to a liquid always raises the boiling point temperature of the resulting solution—;that's called boiling point elevation. This occurs because adding a solute to a liquid always lowers the vapor pressure of the solution—;vapor pressure depression. Recall two more things: 1) The vapor pressure of a liquid always gets higher with higher temperature, and 2) a liquid boils when its vapor pressure is equal to the atmospheric pressure. So if the vapor pressure of a solution is lowered by the addition of a solute, we have to heat the solution to a higher temperature before the vapor pressure equals the atmospheric pressure and the solution will boil again.
111 F, T Divide and conquer. The Lewis dot structure for BF3 is
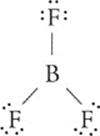
Now, count the groups of electrons around the central atom (B), keeping in mind that every pair of nonbonding electrons, every single bond, every double bond, and every triple bond counts as one group. So here, boron is surrounded by three groups of electrons. Any atom that is surrounded by three groups of electrons has an sp2 hybridization and a trigonal planar geometry. Of course, the second statement is true because this B doesn't have a stable octet—;it has only 6 electrons.
112 T, T Divide and conquer. Like all peroxides, hydrogen peroxide is a good oxidizing agent. It is also true that the hydrogen atoms in H2O2 have a +1 oxidation number. However, the oxidizing tendency of this molecule is not due to the H, but rather to the fact that each O has a –1 oxidation number, instead of the usual –2.
113 F, F Divide and conquer. Both statements are false. There are no ideal gases—;end of story. Furthermore, H's in H2 are bonded together via a covalent bond. Hydrogen bonding refers to a specific dipole interaction between two or more different molecules where an H covalently bonded to an F, O, or N is electrostatically attracted to an F, O, or N on another molecule.
114 T, T, CE Divide and conquer. Both statements are true. In an ordinary electrochemical cell, chemical reactions produce electricity (like in a battery). In contrast, in an electro-lytic cell, electrical energy is added to produce a chemical reaction (like a battery being recharged). This is because the chemical energy of the products is greater than that of the reactants.
115 T, F Divide and conquer. Over ![]() of the mass of the average human is oxygen. However, most oxygen atoms in the bloodstream, over 99.99 percent of them, are in the form of H2O, not as O2. The first statement is true, but the second is false.
of the mass of the average human is oxygen. However, most oxygen atoms in the bloodstream, over 99.99 percent of them, are in the form of H2O, not as O2. The first statement is true, but the second is false.
116 T, T Divide and conquer. Strong acids and strong bases are those that undergo 100 percent dissociation in water.
The strong acids are HCl, HBr, HI, HNO3, H2SO4, and HClO4.
The strong bases are LiOH, NaOH, KOH, RbOH, and CsOH.
Mixing any acid with any base will produce a neutralization reaction, regardless of whether the acid and base are strong or weak.
PART C
33 D First, draw the Lewis dot structure for XeOF4.
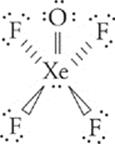
Now, count the groups of electrons around the central atom (Xe) keeping in mind that every pair of nonbonding electrons, every single bond, every double bond, and every triple bond counts as one group. So here, Xe is surrounded by six groups of electrons. Any atom that is surrounded by six groups of electrons has an sp3d2 hybridization and an octahedral geometry. However, because the question asks about the molecular shape (as opposed to the geometry), we look at the arrangement of the surrounding atoms. In this case, the F would make a flat square around the Xe with the oxygen atom lying directly above. This traces out a square pyramid (choice (D)).
34 A After examining the periodic table, realize that the full atomic symbol for this isotope of technicium is
![]()
Remembering that the superscript (the mass number) represents the total number of protons and neutrons, and the subscript (the atomic number) represents just the number of protons, has 43 protons and 99 – 43 = 56 neutrons—;choice (A).
35 D Recall that the six strong acids are HCl, HBr, HI, HNO3, HClO4, and H2SO4. Phosphoric acid, H3PO4, is a weak acid.
36 B First, the correct abbreviation for heat is Q. So we can eliminate choices (C), (D), and (E). During a phase change, the temperature of a substance remains constant. Therefore, choice (A) must be wrong because it has a term for changing temperature, ΔT. In fact, choice (A) is the equation used to determine the change in temperature when heat is added or removed from a substance that isn't undergoing a phase change. Choice (B) is the correct choice, where Q is the heat added or removed, n is the number of moles of substance, and ΔH is the heat of phase change, a quantity that is unique for every substance.
37 E First, according to the periodic table, Cr has 24 electrons. We can eliminate choices (B) and (C) because those configurations have 25 electrons. Choice (A) is wrong because the 3s subshell is already accounted for in the [Ar] core—;i.e., [Ar] stands for 1s2, 2s2, 2p6, 3s2, 3p6. Now, according to the Aufbau principle, we completely fill subshells before moving up to the next higher one. So the best answer would appear to be choice (D). However, remember that completely half-filled and filled d subshells bestow extra stabilization to an atom. Therefore, Cr and Cu actually violate the Aufbau principle and promote a 4s electron to become [Ar] 4s13d 5 and [Ar] 4s13d10, respectively. Choice (E) is the correct ground state configuration for Cr.
38 D At 25°C, pH + pOH = 14 for any solution. Therefore, if the pH is 10 for this solution, the pOH is 14 – 10 = 4. Taking the negative antilog of 4 gives 10−4, choice (D).
39 D First, find the number of moles of Br−.

Then figure out how much 0.05 moles of Br− weighs.
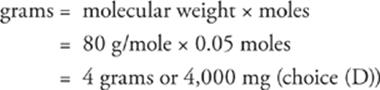
40 B This is another math problem, but this one involves the ideal gas law. A couple of things first: 1) Since we don't have a calculator, let's round off the value of R to 0.1 L•atm/mol•K, and 2) remember that T must be in K, not °C, for PV = nRT to work correctly. So

41 E Recall that when ΔG is negative, a reaction is spontaneous, and when ΔG is positive, a reaction is nonspontaneous. The question indicates that the reaction in question is endothermic—;i.e., ΔH is positive. Looking at the equation provided, the effect that decreasing T has will depend on the sign of ΔS. But the question doesn't tell us the sign of ΔS, nor can we figure it out on our own. Therefore, there is no way to make any conclusions—;choice (E).
42 B First ionization energy is a periodic trend that increases up and to the right on the periodic table. After looking at the position of these elements on the periodic table, carbon is clearly the best answer—;choice (B).
43 A Lewis acids are molecules/ions that can accept a pair of nonbonding electrons to form a covalent bond. Not surprisingly, molecules/ions that contain an atom with an incomplete octet are good Lewis acids. The only choice that has an atom without a stable octet (or bitet in the case ofH) is the carbon in choice (A).
44 D According to the reaction, 6 Ca atoms are present in the reactants. Therefore, 6 CaSiO3 must be present as products, choice (D).
45 E Given that the molecular weight of AgCl is 143.4 g/mol, the number of moles of AgCl precipitated is
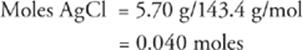
Since the molar ratio of Ag+ in AgCl is 1, the number of moles of Ag+ in the original solution was also 0.040. Therefore, the concentration of the original solution was

46 A Choices (B), (C), (D), and (E) are all true statements. Choice (A) is a false statement—;the vapor pressure of a substance depends only upon 1) the substance's temperature and 2) its mole fraction when it's in solution (see the colligative property of vapor pressure depression).
47 B Since these are all ionic compounds, electrostatic forces can be assumed to be entirely responsible for the cohesive forces on the lattice. According to Coulomb's law
F = Kq1q2/r2 where Energy = Kq1q2/r
Since the charges for all of the ion pairs given in the choices (q1 and q2) are ±1, it is the internuclear distance, r, of each ion pair that is the determinant factor. According to the equations above, the smaller the r, the greater the energy. So using the periodic trend in atomic/ion size, LiF (choice (B)) is the ion pair with the smallest internuclear distance.
48 C Positron emission is a type of beta decay. During beta decay, nuclear mass remains constant. Therefore, choice (C) is the only possible answer.
![]()
Conserving mass (superscript) and charge (subscript) gives choice (C).
49 C Recall the solubility rules. (A) and (B) are incorrect because neither NaCl nor BaOH are insoluble and therefore would not precipitate out of the aqueous solution. Because lead salts are insoluble except for their nitrates and perchlorates, lead iodide would precipitate out of solution as (C) depicts. Answer choices (D) and (E) are examples of oxidation-reduction reactions.
50 A Cell diagrams are read as
Anode|Anodic Solution|Cathodic Solution|Cathode
For any electrochemical or electrolytic cell, oxidation occurs at the anode and reduction occurs at the cathode (remember AN OX and RED CAT). Therefore, the correct choice must be an oxidation. This eliminates choices (B) and (C). We can also eliminate choices (D) and (E) because Cl is never allowed to enter the anodic side of the cell. Therefore, choice (A) is the correct answer.
51 E According to Le Chatelier's principle, the direction in which an equilibrium is disturbed can be predicted if ΔHr × n is known (eliminate choice (B)). A straightforward way of solving this is to write “HEAT” into the reaction either as a reactant for endothermic reactions or as a product for exothermic reactions. Here, the reaction is exothermic, so
A ![]() B + C + “HEAT”
B + C + “HEAT”
Then, since temperature is a measure of “HEAT,” increasing T, or “HEAT,” would be expected to shift the system to the left. Choice (E) is the answer.
52 D Recall some fundamental solubility rules.
• All group 1 metals and salts are soluble.
• All and salts are soluble.
• All silver, lead, and mercury salts are insoluble.
Therefore, the addition of Li+, NH4+, or Cs+ would not produce a precipitate with either acid (eliminate choices (A), (B), and (C)). Silver, Ag+, would form precipitates with both acids (eliminate choice (E)). Ba2+ is somewhat unique, even among other group 2 elements, because BaCl2 is soluble while BaSO4 is not choice (D).
53 A Writing equilibrium expressions is a three-step process.
1. First, ignore any molecule that is in the solid (s) or liquid (l) phases.
2. Second, write K = [products]/[reactants]
3. Third, all coefficients in front of molecules become exponents in the equilibrium expression.
Therefore, the correct choice is (A).
54 D A conjugate acid/base pair is a set of 2 molecules/ions that have identical molecular formulas, except that one of them has one more H+ than the other. Only choice (D) represents a true pair of conjugates
55 B The starting solution has a pH of 3.5. Therefore, the starting solution must be acidic. We can eliminate choices (C) and (D) because they are bases. The subsequent titration experiment revealed a single equivalence point, so the acid in question must be monoprotic—;eliminate choice (E). Since the equivalence point comes at pH 7, the unknown acid must be strong. HCl is the only monoprotic strong acid given, choice (B).
56 E Statements (A) through (D) can all be determined with data obtained from a titration experiment.
57 A The phase changes associated with the terms are
deposition (gas → solid)
sublimation (solid → gas)
liquefaction (gas→ liquid)
fusion (solid → liquid)
vaporization (liquid → gas)
58 D The molecular formulas associated with the IUPAC names for organic molecules are
|
methane: |
CH4 |
|
ethane: |
CH3CH3 |
|
methylethane: |
does not exist in the IUPAC system |
|
propane: |
CH3CH2CH3 |
|
isopropane: |
does not exist in the IUPAC system |
59 E First, figure out the concentration of [H+] at pH 1 and pH 3.
pH = 1; [H+] = 0.1 M pH = 3; [H+] = 0.001 M
Then realize that 0.001 M is 1 percent of (or 100 times smaller than) 0.1 M. Therefore, 99 percent of the H+ is neutralized when going from pH 1 to pH 3.
60 A Avogadro's number is nothing more than the conversion faction among two measures of mass, atomic mass units, and grams. Just as you can say that there are 2.54 centimeters in an inch, so too can you say there are 6.02 × 1023 amu in a gram.
61 C Process of elimination is the best tool to use here. First, we can eliminate mercury (choice (A)) because mercury metal is a liquid at standard conditions, yet the table indicates that unknown metal #1 is a solid. Second, we can eliminate copper (choice (B)) because copper metal is brownish, yet the table says unknown metal #1 is dull gray. Now, consider the presence of the white oxide coat. The oxide of iron, better known as rust, is orange brown, not white, so eliminate choice (D). Last, the oxide of silver is gray-black, better known as tarnish, so eliminate choice (E). Therefore, zinc is the best choice—;choice (C).
62 E Again, process of elimination is the best tool to use here. First, we can eliminate carbon (choice A) because carbon is not a metal. Second, we can again eliminate copper (choice B) because copper metal is brownish, yet the table says unknown metal #2 is silver-gray. Third, we can eliminate zinc (choice (C)) because it is a fairly reactive metal that always has a whitish oxide coat in air. Fourth, we can definitely eliminate sodium because sodium metal explodes with yellow flame on contact with even plain water, let alone acidic solutions—;the table doesn't report any explosions! Finally, the best choice is silver, a relatively inert metal along with copper, gold, and platinum (it's no accident that these metals are used for jewelry and coinage)—;choice (E).
63 B There are several ways to approach this one. Again, process of elimination is very useful here. Chlorine (choice (A)) and nitrogen dioxide (choice (E)) gases are colored—;greenish and orange, respectively. Therefore, they cannot be the gas in question. Furthermore, it's difficult to choose carbon dioxide (choice (D)) because there are no carbon atoms anywhere in this experiment. Finally, as a rule of thumb, a colorless gas produced from reactions between metals and acids is hydrogen—;choice (B).
64 E This requires a bit of general chemistry knowledge. The colors of the gases are
Cl2—;green
H2—;colorless
O2—;colorless
CO2—;colorless
NO2—;orange/brown
Choice (E) is the best choice.
65 B First, the neutralization reaction that occurs here is
KOH + HI → KI + H2O
The trick is to realize that the number of K's and I's are not changing during the reaction; but since the solutions are being added, the volume is doubling. If the volume doubles, then the initial solution concentrations (0.2 M) are halved (0.1 M).
66 D An O2– ion has gained 2 electrons to fill its outer shell. This gives an O2– ion the same electron configuration as Ne. That is, the 2 are isoelectronic.
67 D The addition of a catalyst to a system at equilibrium has NO effect. That's because all a catalyst does is increase the rate at which a nonequilibrated system reaches equilibrium.
68 A It's important to know the phase of the elements at standard conditions (25°C at 1 atm).
|
Gases: |
hydrogen, helium, nitrogen, oxygen, fluorine, neon, chlorine, argon, krypton, xenon, and radon |
|
Liquids: |
mercury and bromine (choice (A)) |
|
Solids: |
the rest of the elements |
69 C By definition, accuracy is the measure of how close experimental data are to true data, while precision is the measure of how similar experimental data are to one another. Clearly, the student's data is very precise; the values of 1.65, 1.68, 1.71 vary by no more than 4 percent. However, the average value of the experimental data is nearly 50 percent off the true value, meaning these results are not very accurate—;choice (C).