SAT Subject Test Physics (2012)
PART III. PHYSICS TOPIC REVIEW
Chapter 11. THERMODYNAMICS
Thermodynamics is the study of how heat, energy, and work affect a system. Unlike the kinetic theory, which you reviewed in the previous chapter, thermodynamics deals with large-scale changes in a system. There are four basic laws of thermodynamics, but SAT Physics will primarily deal with only the first two and their related processes.
First Law of Thermodynamics
The first law of thermodynamics relates the law of conservation of energy to processes involving heat. According to this law, the change in the internal energy of a system ΔU equals the heat added to the system Q minus the work the system does W.
![]()
If work is done on a system, the value of W is negative. Therefore, you might see the equation as ![]() . If you look at the equation, you can see that it describes that energy cannot be created or destroyed. If the energy of the system changes, the energy of the environment outside the system must also change.
. If you look at the equation, you can see that it describes that energy cannot be created or destroyed. If the energy of the system changes, the energy of the environment outside the system must also change.
Recall that work is done when a force is exerted to move a body some distance. In a thermodynamic system, work is often done on or by a gas in a cylinder confined by a piston. When the piston is pushed down, it does work on the gas. When the gas is heated, it will expand and push the piston upward, thereby doing work on the piston.
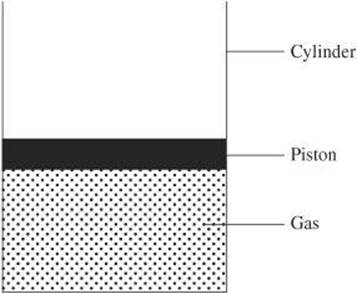
Example:
A system does 30 J of work when 80 J of heat are added to it. What is the change in the internal energy of the system?
![]()
Special Thermodynamic Processes
The first law of thermodynamics explains that a system’s internal energy can be changed by the transfer of energy either as heat, work, or some combination of the two. Several processes are special conditions of the first law.
Adiabatic A process is said to be adiabatic if it is thermally insulated from its environment. In other words, no energy is transferred as heat. If a change is adiabatic, ![]() , so
, so ![]() . Consider a gas in a cylinder confined by a piston. The temperature of the gas will change as the internal energy changes. Therefore, the gas will warm up as the gas is compressed and cool down as the gas expands. Adiabatic processes are considered theoretically only because perfect thermal insulation does not exist. For compression or expansion that occurs rapidly, however, the process approaches adiabatic conditions because heat takes time to flow.
. Consider a gas in a cylinder confined by a piston. The temperature of the gas will change as the internal energy changes. Therefore, the gas will warm up as the gas is compressed and cool down as the gas expands. Adiabatic processes are considered theoretically only because perfect thermal insulation does not exist. For compression or expansion that occurs rapidly, however, the process approaches adiabatic conditions because heat takes time to flow.
Isothermal In an isothermal process, the internal energy remains constant, and there is no change in temperature. As a result, ![]() , so
, so ![]() . The heat flow and the work done exactly balance each other. Any energy added to a system as heat is removed as work done by the system. Any energy added to the system by work done on it is removed as heat.
. The heat flow and the work done exactly balance each other. Any energy added to a system as heat is removed as work done by the system. Any energy added to the system by work done on it is removed as heat.
Isovolumetric No work is done in an isovolumetric process. As a result, ![]() , so
, so ![]() . Any energy added to the system as heat increases the internal energy. Any energy removed from the system as heat decreases the internal energy.
. Any energy added to the system as heat increases the internal energy. Any energy removed from the system as heat decreases the internal energy.
Isolated System A system is said to be isolated if it experiences no heat or work interactions with the surrounding environment. There is no change in the internal energy for a closed system.
Refrigeration and Heat Engines
In a cyclic system, the change in internal energy is zero, and the final and initial values of internal energy are the same.
![]()
The process is somewhat similar to an isothermal process. However, the process repeats itself.
A refrigerator takes advantage of a cyclic process. Heat is transferred from the interior of the refrigerator to an evaporating refrigerant (Qc). In another part of the system, energy is transferred as heat from a hot condensing refrigerant to the air outside the refrigerator (Qh).
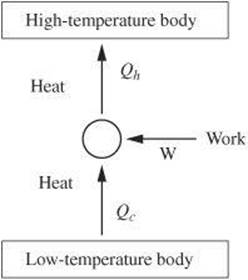
The difference between the two values is the net heat, and, therefore, the net work done during each cycle of the process.
![]()
A heat engine is a device that converts heat to mechanical energy by doing work. It uses a process that is opposite that of a refrigerator. The basic concept behind a heat engine is that mechanical energy can be obtained from thermal energy when heat is allowed to flow from a high temperature to a low temperature. During this process, some energy is transformed to mechanical work as summarized in the following diagram.
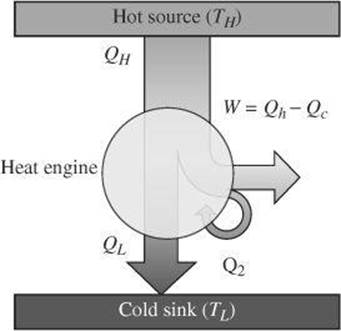
The amount of work the heat engine can do depends on the difference between the amount of energy transferred as heat into the engine and out of the engine.
No cyclic process can convert all of the energy transferred as heat into work. In addition, it cannot transfer energy as heat from a cooler body to a warmer one without work being done. The efficiency of an engine (eff) is a measure of the useful energy taken out of a process compared with the total energy put into the process
![]()
which can be rewritten as
![]()
Another way to look at this equation is as follows:
![]() - energy removed as heat/energy added as heat
- energy removed as heat/energy added as heat
Multiply the resulting decimal from each equation by 100% to find the efficiency as a percentage. Now you can see that a heat engine would only be 100% efficient if no heat were removed. Because this is not possible, all heat engines have an efficiency that is less than 100%.
Example:
A heat engine receives 160 J of energy from a hot reservoir, does work, and exhausts 50 J of energy into a cold reservoir. How much work is done by the engine? What is its efficiency?
![]()
The percent efficiency is Wnet/Qh × 100%.
![]()
Second Law of Thermodynamics
The second law of thermodynamics deals with entropy, which is a measure of the disorder of a system. According to the second law, spontaneous processes that proceed in an isolated system lead to an increase in entropy. What that means is that an isolated system will naturally change toward a state of greater disorder. Unlike other quantities in physics, entropy is not conserved in natural processes. Entropy in the universe is increasing.
When dealing with entropy S, it is the change rather than the absolute amount that is important. The change in entropy ΔS can be described by the following equation, in which Q is the amount of heat added to the system and T is the kelvin temperature.
![]()
Heat Transfer
Throughout this chapter, you have been considering heat transfer into and out of systems. Heat is transferred in three ways.
Conduction If you put a metal spoon in a bowl of hot soup, the spoon will eventually become hot. The method through which heat is transferred to the spoon is by conduction, which is the transfer of heat between materials that are in contact with one another. Materials through which heat passes easily, such as metals, are known as conductors. Materials that resist the transfer of heat are known as insulators. Wood, rubber, and air are good insulators.
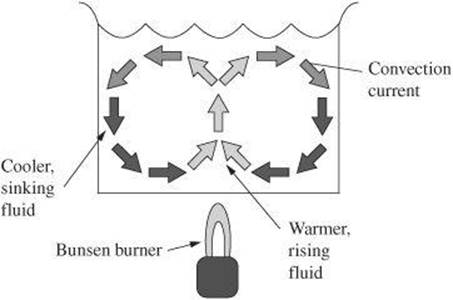
Convection When a fluid is heated, the particles move faster and farther apart. As a result, the density of a fluid decreases. A heated fluid therefore rises and the cooler fluid above it sinks into its place. The cooler fluid then becomes heated and the process repeats to produce a convection current. Eventually, heat is transferred throughout the fluid through this method, which is known as convection.
Radiation Heat from the sun is transferred to Earth through the vacuum of space. Radiation is a method of heat transfer that can occur through a vacuum. This method of heat transfer involves electromagnetic waves, which will be discussed in more detail in Chapter 16. The warmth you feel from a campfire results from radiation.
Test-Taking Hint
If you are unsure of an answer, try to rule out any answers you know to be incorrect. For example, if you know that internal energy increases or decreases, you can eliminate some answers accordingly. Once your possible choices are reduced, you can try to figure out the correct one.
REVIEW QUESTIONS
Select the choice that best answers the question or completes the statement.
1. A total of 110 J of work is done on a gaseous refrigerant as it is compressed. If the internal energy increases by 85 J during the process, how much energy is transferred as heat from the refrigerant?
(A) –25 J
(B) –10 J
(C) 0 J
(D) –85 J
(E) –195 J
2. What is the efficiency of a heat engine that receives 200 J of energy from combustion and loses 130 J as heat to exhaust?
(A) 0.159
(B) 0.350
(C) 0.550
(D) 0.650
(E) 0.800
3. What happens to a gas that is compressed rapidly by a piston? [Assume friction is negligible.]
(A) its temperature decreases.
(B) its temperature increases.
(C) its temperature remains the same.
(D) it does positive work on the piston.
(E) it becomes a solid.
4. Which energy transformation takes place in a heat engine?
(A) mechanical to heat
(B) electrical to heat
(C) thermal to mechanical
(D) chemical to heat
(E) thermal to electrical
5. The first law of thermodynamics is an application of which other law?
(A) the law of universal gravitation
(B) the law of harmonies
(C) the law of entropy
(D) the ideal gas law
(E) the law of conservation of energy
6. Which of the following is a consequence of the second law of thermodynamics?
(A) the change in the internal energy of a cyclic process is zero.
(B) heat can be transferred through a vacuum by electromagnetic waves.
(C) the acceleration of an object depends on its mass and the net force exerted on it.
(D) heat does not flow naturally from cooler substances to warmer ones.
(E) energy is neither created nor destroyed when work is done on a system.
7. A scientist finds that ![]() . Which type of process is the scientist observing?
. Which type of process is the scientist observing?
(A) isothermal
(B) isolated
(C) adiabatic
(D) isovolumetric
(E) reverse
8. Fifty joules of heat flow into a system. The system does 70 joules of work. What happens to the internal energy of the system?
(A) it remains constant.
(B) it increases by 120 J.
(C) it decreases by 120 J.
(D) it increases by 20 J
(E) it decreases by 20 J.
Questions 9 and 10 relate to the diagram below, which shows a pot of water being held above a campfire.
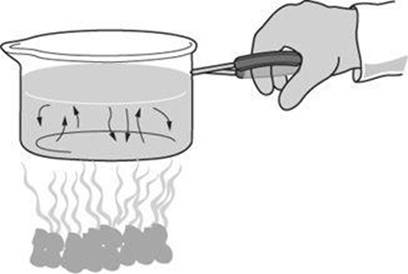
9. Through which process is heat transferred from the fire to the person’s hand?
(A) conduction
(B) friction
(C) convection
(D) radiation
(E) entropy
10. Convection currents can form in liquid water, but not solid metal, because
(A) fluids expand when heated.
(B) electromagnetic waves carry energy.
(C) work is done when heat is transferred.
(D) the specific heat capacity of water is high.
(E) the arrangement of water molecules resists the transfer of heat.
QUESTION ANSWERS AND EXPLANATIONS
1. A Work is done on the gas so the value of W is negative. ![]() . The internal energy increases, so ΔU has a positive value.
. The internal energy increases, so ΔU has a positive value. ![]() . Rearrange the first law
. Rearrange the first law ![]() to solve for
to solve for ![]() .
.
2. ![]() and
and ![]()
![]()
3. B The piston does work on the gas, causing the gas to become warmer. If the process is rapid, the heat cannot escape the system immediately and the temperature of the gas rises.
4. C A heat engine is a device that transforms heat into mechanical energy that can be used to do work.
5. E The first law of thermodynamics is a form of the law of conservation of energy. It states that energy cannot be destroyed and that if the internal energy of a system changes, the energy of the surrounding environment must also change.
6. D Natural processes increase entropy. Adding heat to a system increases the entropy of its particles. Work must be done to cause heat to flow from a cooler substance to a warmer one.
7. C If a change is adiabatic, ![]() . Therefore,
. Therefore, ![]() becomes
becomes ![]() , so
, so ![]() .
.
8. ![]() . The negative sign indicates a decrease.
. The negative sign indicates a decrease.
9. D The hand is not touching the fire and currents do not carry heat to the hand. The process through which heat is transferred from the fire is radiation.
10. A The water at the bottom of the pot is heated, causing it to expand. As the heated water expands, its density decreases. The less dense water rises as the denser water above it sinks, forming convection currents.