Make: The Annotated Build-It-Yourself Science Laboratory (2015)
Part II. Physics
Chapter 3. Atomic Energy
Radiometric Dating
Purpose: This model clock demonstrates how scientists can tell the age of the Earth by examining radioactive ore. The clock can also be used as a timing device.1
Materials: Two straight-sided bottles (such as baby bottles), two rubber stoppers (two-hole), and two very short pieces of glass tubing.
What to Do: Join the two stoppers together with glass tubing through each hole (for baby bottles the stoppers should be #7). Fill one of the bottles with water. Insert the stoppers and the other bottle as shown. Turn the “atomic time clock” over so the water slowly runs into the second bottle.2
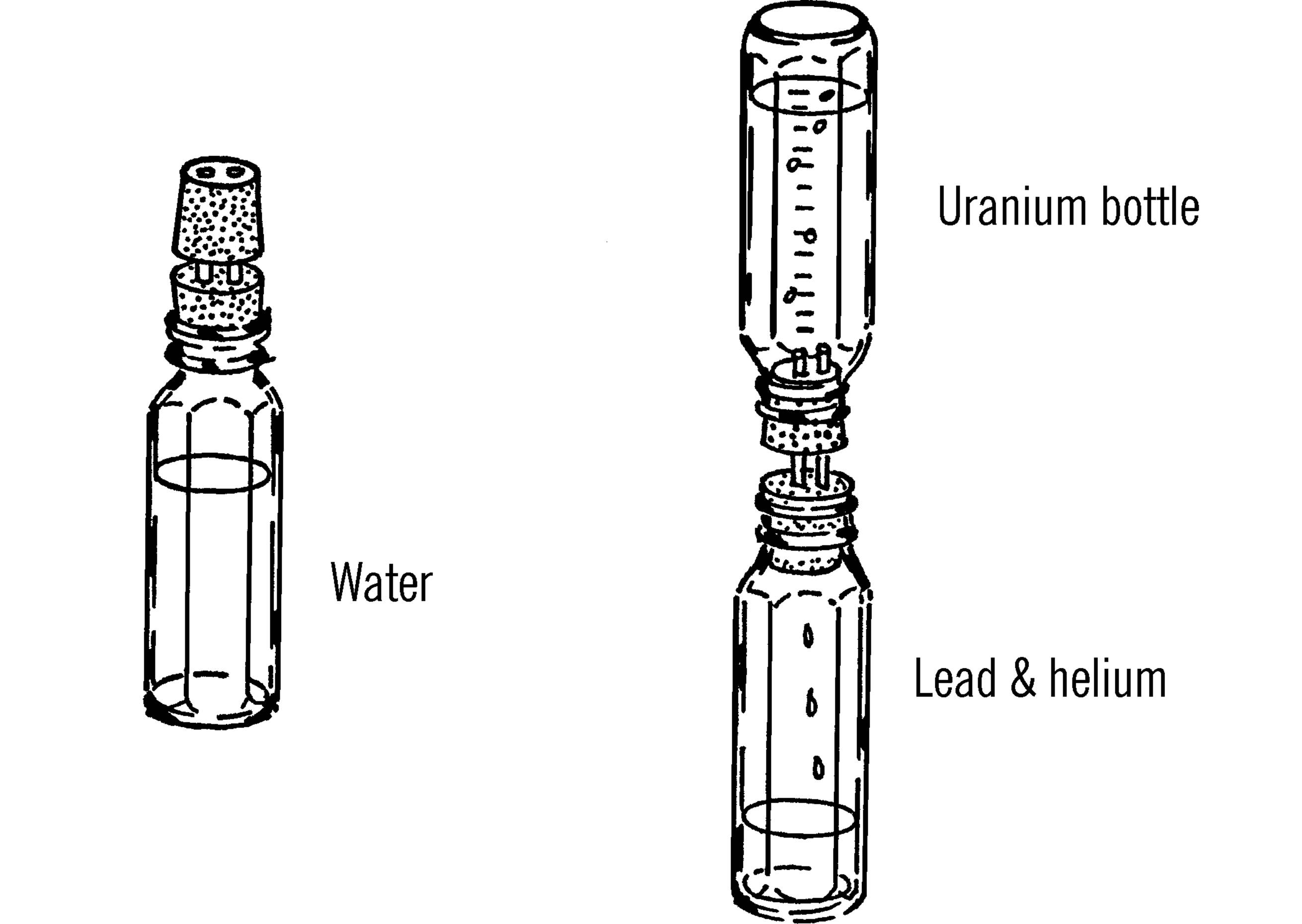
Operation of Equipment: Time how long it takes the water to flow to a depth of one inch, then two inches, etc. If you use a baby bottle, marks are already calibrated in CC’s. You can change your bottle into quite an accurate clock. Place a piece of masking tape alongside the marks on the bottle. Place a pencil mark for the time it takes the water to flow to that height. You will probably have a mark for one minute, two minutes, etc. You might even have short marks for the half minutes between the long marks.
The scientist uses a similar clock to tell the age of rocks. Uranium gives off radioactivity at a rate as regular as water flows. The uranium gradually changes into helium and lead. The scientist notes the percentage or amount of uranium as compared to lead and helium. By knowing the rate of flow or change, he3 can tell quite accurately how old the piece of uranium is.
Mark one bottle uranium. Mark the second bottle lead and helium. Now place another tape on the bottle labeled lead and helium. This time, instead of minutes, label the marks in thousands of years.
Can You Work Like a Scientist?
1. Have a student leave the room. This student represents the scientist who wasn’t even alive at the time these rocks were formed. At some time after the student has left the room, start your water atomic clock. Note the time you started the water (or uranium) flowing. Call the student back into the room and have him tell you exactly how long ago you started the water flowing. This would be the same as the scientist telling the age of the rock.
2. Why do you use two-hole rubber stoppers? Can you make a clock using white sand?
3. Heat some glass tubing and pull it into a narrow opening. If you used this glass tubing with a narrow opening, would your time clock run slower or faster? Do certain radioactive materials have a slower rate of flow than others?
Spinthariscope
Purpose: This is a small instrument for watching atoms of radioactive materials bombarding a phosphorescent screen.
Materials: Small cardboard box (about 3” × 3” × 3”), cardboard tubing, small glass plate to fit into the bottom of the box, small double convex lens (magnifying lens), zinc sulfide, and a source of radioactive material.4
What to Do: Remove the radioactive paint from the numbers of an old alarm clock5 by scraping the paint with a sharp knife. Place the small bits of radioactive material on a clean piece of glass. The glass should be small enough to fit in the bottom of the cardboard box. You can cut glass by following the directions in the chemistry section.
Add about a gram of zinc sulfide powder to the glass plate. Mix this powder and the radioactive material together. Grind the two powders so the mixture contains very small bits of both materials. Remove the mixture from the glass and coat the glass with a layer of shellac.6 While the shellac is still tacky, the mixture is sprinkled over the glass surface. Care should be taken to spread the mixture evenly. Don’t handle the radioactive material with your bare hands. Wash thoroughly after working with radioactive materials.
The glass is then glued with airplane cement7 to the bottom of the box, as shown. Be sure that the coated side of the glass plate is up. Allow the coating on the glass to dry completely.
Cut a hole in the part of the box opposite the glass plate and mount a lens much the same way as you did with the telescope. Over this hole and lens glue a piece of cardboard tubing about an inch in length.
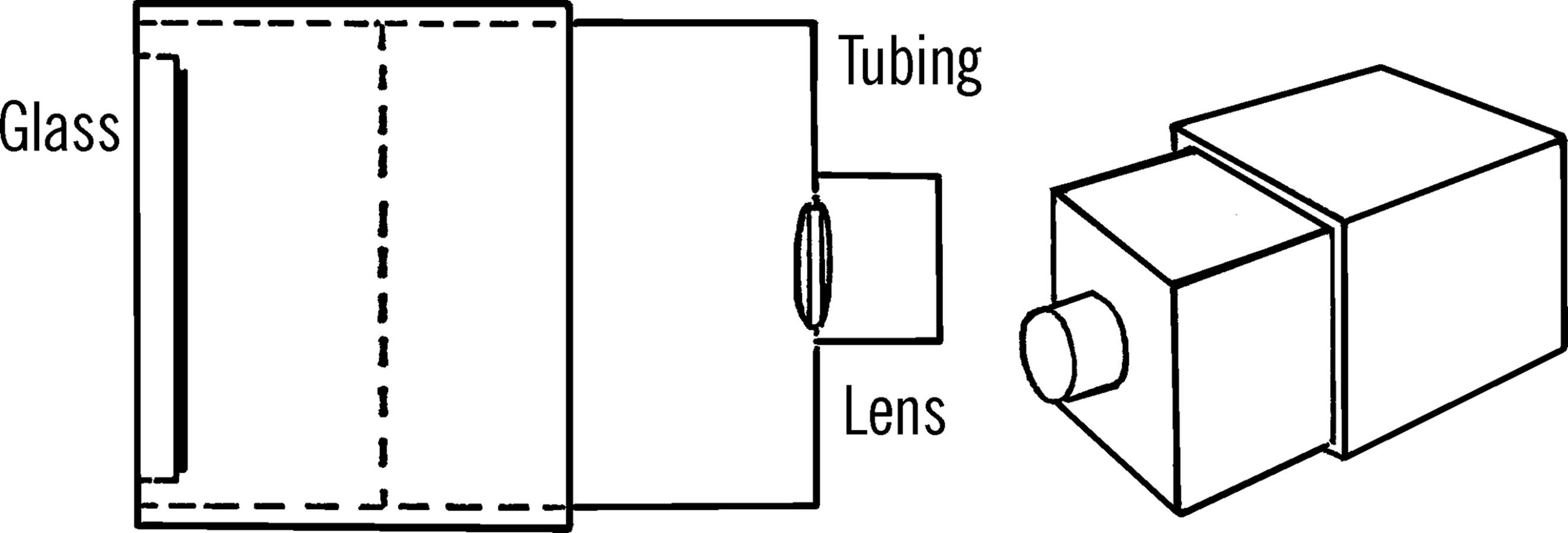
Operation of Equipment: The spinthariscope works the best under total darkness. Therefore, use the instrument in a darkened closet or a room without outside windows. Look through the cardboard tubing toward the glass plate. Focus the lens by moving the top and bottom of the box apart. It probably will take several minutes for your eye to become adjusted to the darkness. Then, you should see bright flashes as the radioactive atoms throw off bits of themselves and slowly change from one element to another.
Modern Safety Practice
Low-level radioactive sources are potentially hazardous, particularly if ingested. Read Note 28 in Appendix E about radioactive sources.
Can You Work Like a Scientist?
1. When your spinthariscope stops twinkling in the dark, the radioactive materials have completely decomposed into non-radioactive materials. Do you notice any weakening of your spinthariscope over a period of a week? A month? How long will your spinthariscope continue to work?
2. Can you devise a way of measuring the strength by counting the number of flashes in a certain time period? Could you observe the flashes under a microscope?
Dosimeter
Purpose: A dosimeter is used to detect the amount of radiation to which a person or area is being exposed. A dosimeter such as the one described can be used to determine the safe limits when working with radioactive material or it can be used to detect radiation levels after a nuclear bomb has been exploded in the area.
Materials: Aluminum foil, nylon thread, large drinking glass, ruler, Scotch tape, and the lid from a tuna or similar-size can.
What to Do: Wrap a piece of aluminum foil around the outside and bottom of the glass. Remove the glass and then carefully work the aluminum form into the inside of the glass. The aluminum should be carefully worked with your fingers and the end of a pencil so that it takes the form of the inside and the bottom of the glass.
Remove the aluminum from the glass. Measure down two inches and then cut a one inch square window out of the aluminum. Cut a similar window on the other side of the aluminum form so the two windows are in line. Put the aluminum back into the glass and check to see that the two windows line up A.
Cut a lid off a tuna can. Measure one inch from the center of the tin can lid and punch two small holes side by side as close together as possible. Measure one inch from the center of the lid just opposite these holes and make two similar holes B.
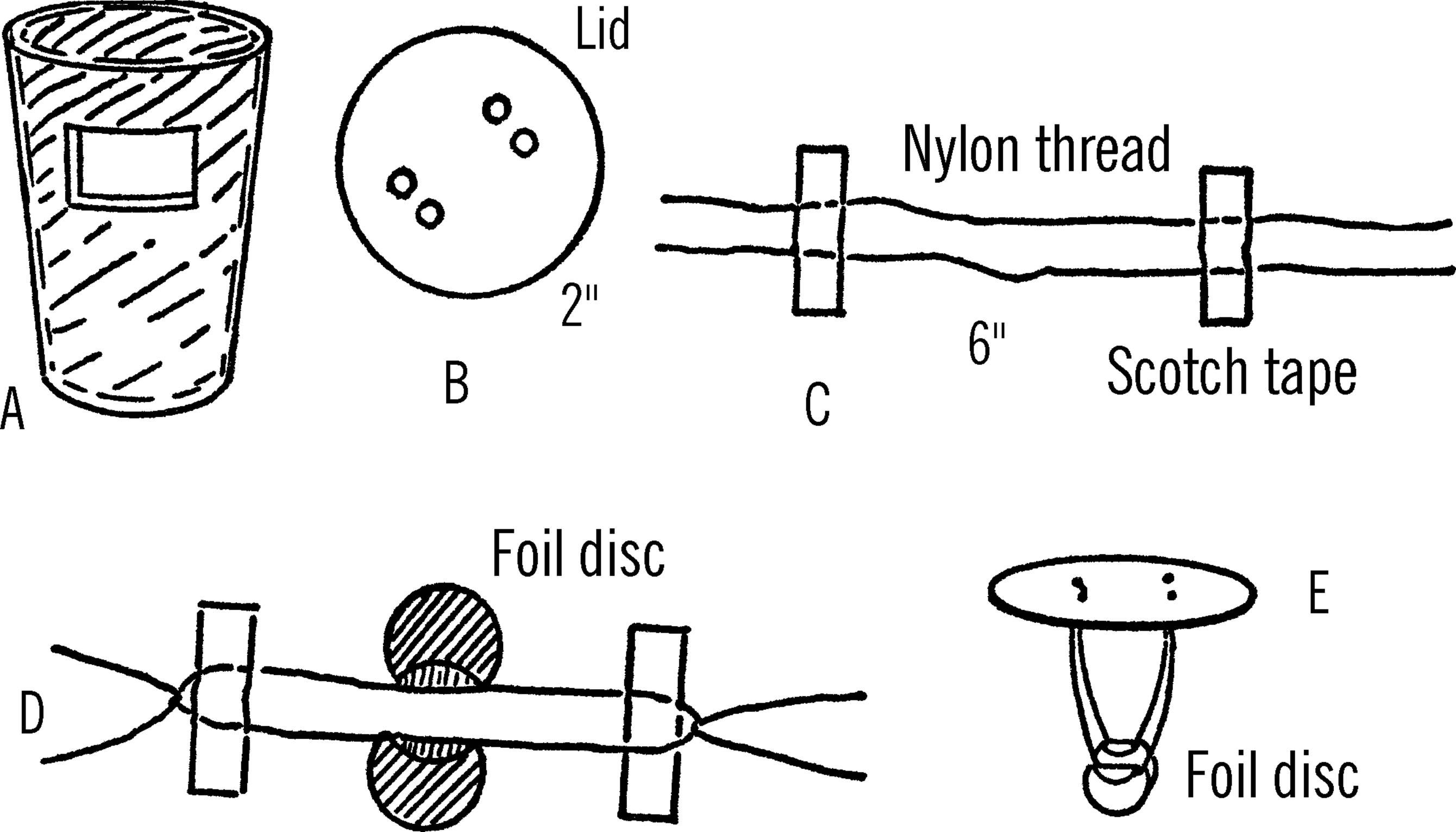
Lay two pieces of nylon thread on a table. Do not touch the thread near the middle. The moisture from your hands will affect the results. Measure three inches from the middle of the two threads and fasten the threads down to the table with Scotch tape. Measure 6 inches from this spot toward the middle of the thread and place another piece of Scotch tape over the two threads C. Cut out two aluminum circles about the size of a quarter. Slide one of the aluminum circles under the thread between the two pieces of Scotch tape. Carefully bend the edge of the disc over the thread. Do the same with the other thread and aluminum disc.
Tie a knot with the nylon threads at each end of the pieces of Scotch tape. The knot should be retied several times so that it won’t come undone later D. Next, slip the two loose ends of the threads through one set of two holes in the lid. Immediately tie the two ends so that the knot fastens firmly down near the lid. Remove the Scotch tape. Repeat the steps with the other two loose ends and the other set of holes in the lid.
When you finish, your two discs of aluminum should be suspended from the lid as shown E. The discs can be slightly moved so that they will match up. Care should be taken not to touch the thread upon which the aluminum circles hang.
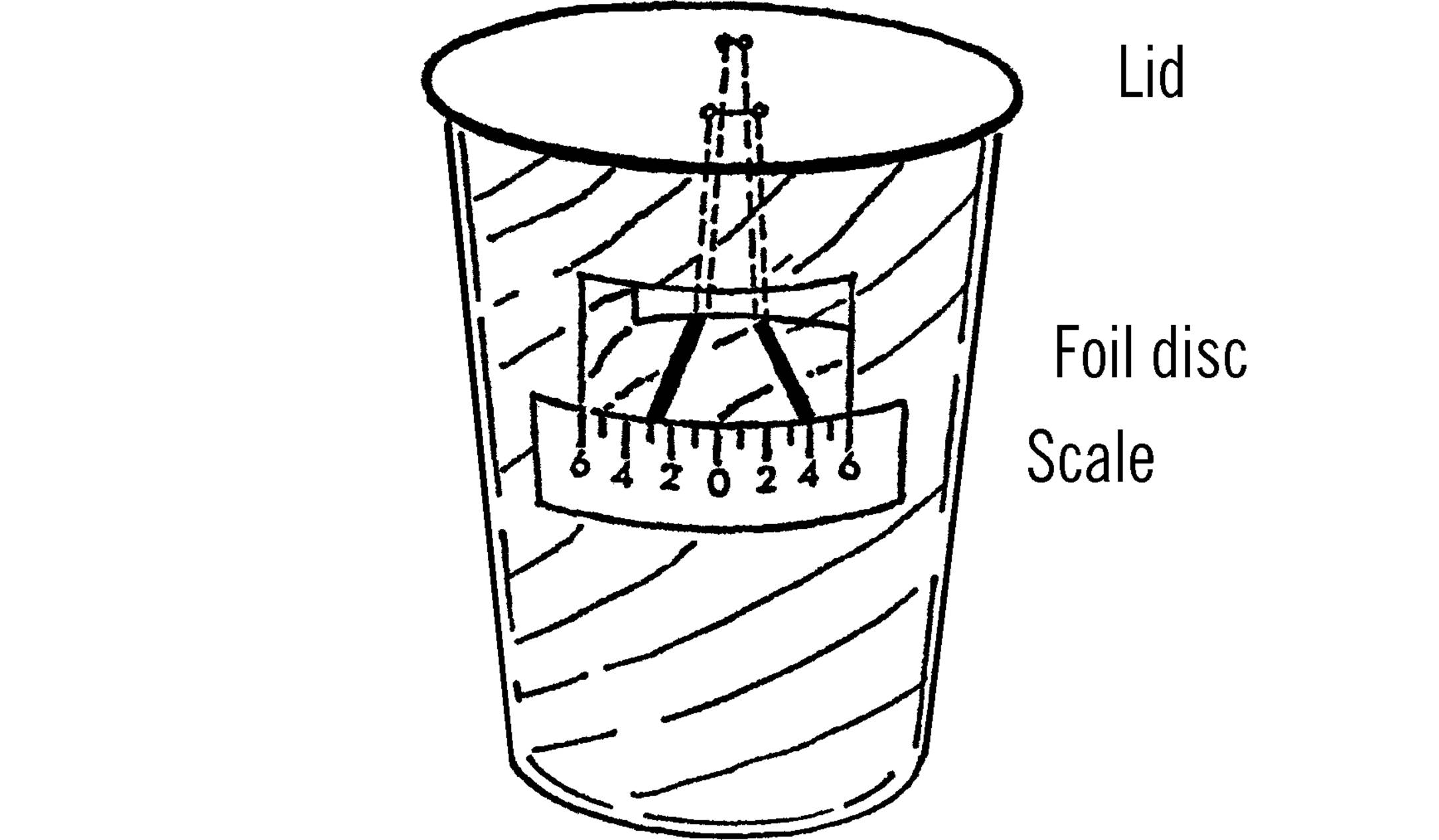
Operation of Equipment: The two aluminum circles suspended from the lid are really a simple type of electroscope. If you bring a material which is charged with static electricity near the piece of foil, the foil becomes charged. Since the charge is the same on both discs, the two circles repel or push each other away. If the lid is carefully lowered over the glass containing the aluminum liner, the two discs will retain their charge. This can be seen through the two windows cut in the aluminum. The lid should be turned so that the discs run perpendicular to the windows. In that way it is easy to tell if the discs are still separated. A scale should be made as shown. This scale is then glued on the outside of the glass just under the window. It is important that the distances on the scale be accurate. Each mark is exactly 2½ mm. apart. Thus the entire scale from 6 to 0 and then to 6 is 30 mm. The scale should be adjusted so that the bottom of each charged disc is the same distance from the 0 mark.
The discs are charged by lifting the lid up and bringing a charged material, such as plastic or a black rubber comb, nearby and touching the discs. Be sure the discs remain charged before you lower them into the glass. The discs should be at least one-half inch apart.
If little or no radiation is present, the discs will remain apart for fifteen or more minutes. If radiation is present, the discs will discharge their static electricity and come together much sooner.
When the discs are placed in the glass, the viewer should mark down the number indicated by the bottom of the disc. The time that it takes the disc to move one mark closer together is then recorded in seconds. The safety limit is then figured by multiplying this time in seconds by ten. The resulting answer is the number of hours a person could stand the degree of radiation present before a fatal dose had accumulated.
Thus, if it takes twenty seconds for the disc to drop from four to three, the time limit would be ten times twenty or two hundred hours.
Modern Safety Practice
This dosimeter is sufficient to demonstrate its principle of operation. However, when human safety is a factor, you must instead use certified and calibrated instrumentation that is designed specifically for radiation safety.
Can You Work Like a Scientist?
1. Dry air is a good insulator and does not conduct static electricity very well. Would your dosimeter be accurate if you had a lot of moisture present?
2. Moisture in the air makes air a better conductor of static electricity. Thus a static electricity charge does not build up as much. Why should you be sure not to touch the nylon thread between the lid and the aluminum disc?
3. Does it take as long for the discs to discharge outside of the glass as inside the glass? What purpose does the aluminum foil have inside the glass?
4. Radiation turns the air into a good conductor of electricity. Why does increased radiation bring the discs closer together?
5. Moisture will affect the accuracy of this instrument. How can you protect your dosimeter from moisture?
Diffusion Cloud Chamber
Purpose: The cloud chamber is used for detecting and identifying radioactive particles and rays such as Alpha particles, Beta particles, and Gamma rays.
The vapor trails of these radioactive materials can be seen when the chamber is operating properly.
Materials: Gallon jug, aluminum cake tin, 7” × 7” plate of window glass, a ¾” × 48” strip of 24-gauge copper, black felt or black enamel paint, sponge, 240-volt photoflash battery, alcohol (or ditto fluid), 5-pound block of dry ice, two pieces of insulated wire, about 4’ of weather stripping, and a radioactive source.8
What to Do: Cut the top and the bottom off the jug with the bottle cutter (see chemistry section). Glue a piece of weather stripping around the top and the bottom on the outside of the jug. The stripping should stick just beyond the edges of the glass so that it can form a seal.
A copper strip is fitted around the outside of the jug. The ends of the strip are soldered together. The strip is positioned about one inch from the top of the jug. A second copper strip is fitted and soldered around the inside of the bottom of the jug.9 This strip should be positioned about an inch and a half from the bottom edge.
The bottom of the aluminum cake or pie tin is either painted black or covered with black felt. When the jug is placed on the aluminum pie tin, the weather stripping should form a seal around the bottom.
The sponge should be cut in strips and glued around the inside of the jug opposite the upper copper band. This sponge is used to hold a supply of alcohol during the operation of the chamber.
A wire is connected to the inside of the lower copper band. The wire is run through the stripping around the bottom of the jug and is connected to the negative pole of the battery. A second wire is run from the positive pole and is connected to the aluminum tin.
The radioactive source is fastened or glued to the point of a thumbtack. Radium can be scraped off a radium dial wristwatch or clock. The tack containing the radioactive sample is then placed in the center of the aluminum tin.
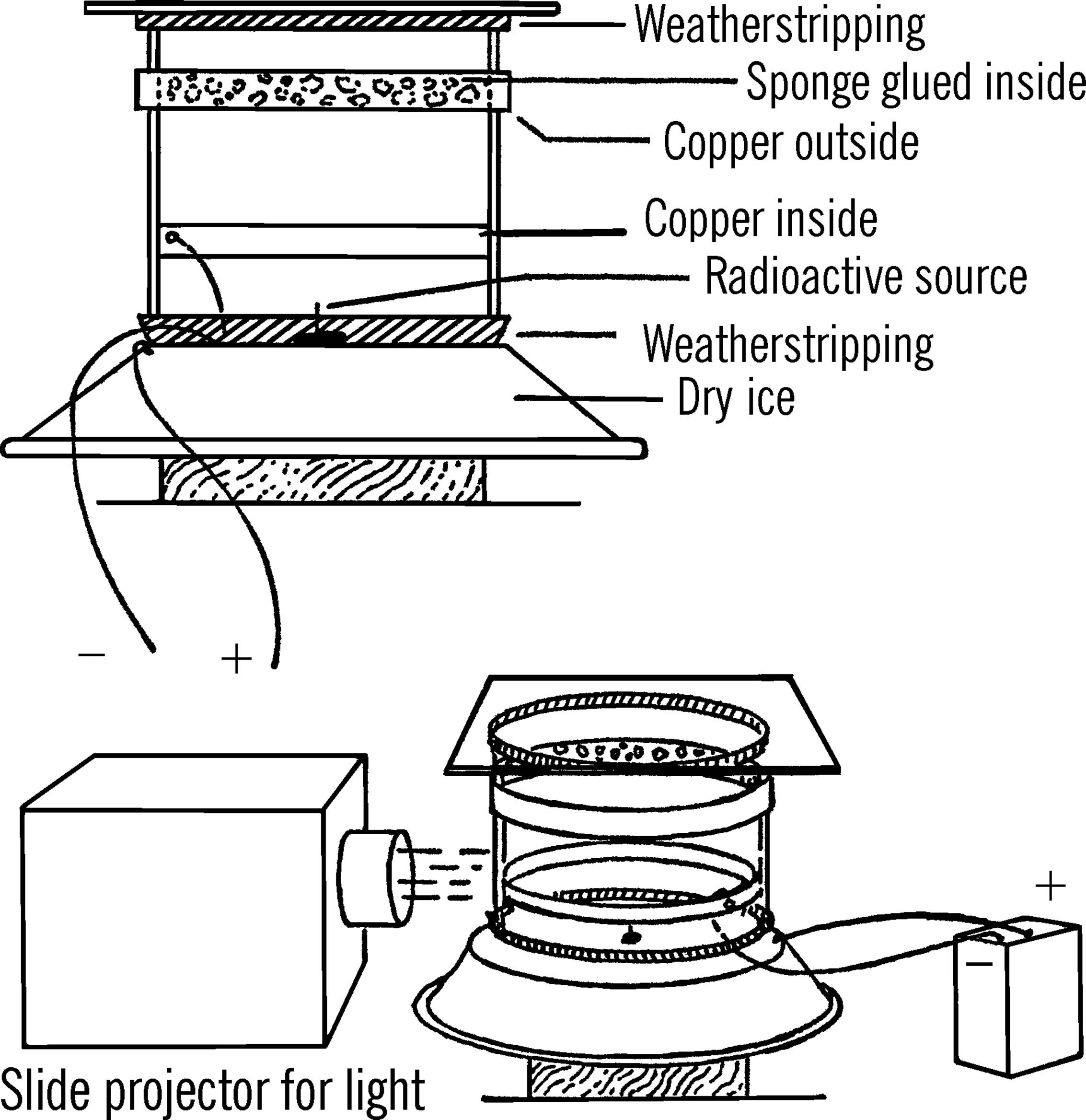
Operation of Equipment: Place a block of dry ice on a plywood board. Set the aluminum tin over the dry ice. The bottom of the tin should be in contact with the dry ice. After the radioactive source has been placed in the center of the bottom of the aluminum cake tin, lower the jug down on the tin. The weather stripping around the bottom of the jug will form a tighter seal if you coat the stripping with Vaseline. Pour a small amount of alcohol around the sponge.
Some of the alcohol will drip down to the bottom of the container. The glass plate should be placed over the top of the jug. Again, Vaseline can be used to make a seal between the stripping and the glass plate. Shine a light source directly across the jug and about two or three inches above the surface of the aluminum tin. A flashlight or slide projector provides a good source of light.10 Finally, the wires should be connected to the battery.
Modern Safety Practice
1. Dry ice can cause severe frostbite injuries. Handle only briefly, using oven mitts or a towel to protect your hands. See Note 14 in Appendix E about safety with dry ice.
2. Avoid directly touching the radioactive source and wash your hands thoroughly after. Read Note 28 in Appendix E about radioactive sources and safety.
3. Alcohol vapor is flammable. Keep sources of spark and flame away, and have a type ABC fire extinguisher handy.
4. If you are applying a voltage between the copper strips, be sure not to touch the strips or wiring. You could receive a severe electric shock. Additionally, there is some chance of a spark when you connect or disconnect wires under power. Connect the wires to your strips first, and only apply or disconnect power from the other ends of the wire, away from the alcohol vapors.
Theory of Operation: Alcohol evaporates from the sponge and the bottom of the chamber. This forms an alcohol vapor atmosphere. The dry ice cools the air near the bottom of the chamber. The cold temperature of the tin draws the heat energy down the glass. The copper band around the outside of the chamber absorbs heat energy from the room and passes this energy through the glass jug and directly to the sponge. This extra heat energy causes the alcohol in the sponge to evaporate more rapidly. As the vapor continues to evaporate, the atmosphere inside the jug is soon saturated with alcohol vapor. As the vapor diffuses (spreads) throughout the chamber, the vapor molecules near the bottom of the chamber are cooled. Since there are more molecules in the form of vapor than can normally exist at a very low temperature, we say the atmosphere near the surface of the aluminum tin is supersaturated. The vapor molecules will condense on charged particles passing through the supersaturated vapor.
Unfortunately, many air molecules have either lost or gained electrons and therefore have an electrical charge. Thus the vapor molecules are very likely to form on the charged air molecules (ions) as a visible alcohol fog. Dust particles have an electrical charge and also serve as nuclei or centers for condensing alcohol vapor.
Since we want to see the vapor trail of just the radioactive material, it is usually necessary to remove the ions (electrically charged molecules) and dust particles from the air. When the wires forming the aluminum tin and the copper band are connected up to the high-voltage battery, negatively charged particles are attracted to the aluminum base because of its positive charge. Since the copper band has a negative charge, particles with a positive charge are drawn to it. This removes electrically charged material from the lower part of the chamber. We then can see the vapor trails formed by the passage of radioactive materials.
An Alpha particle is really the nucleus of a helium atom. This nucleus is thought to consist of two neutrons and two protons. Since the protons have a positive charge, the Alpha particle is charged positively. As this radioactive particle is given off by the radioactive source, it travels through the chamber at about one-twentieth of the speed of light. This particle attracts electrons from the neutral air molecules, thus giving the molecules an electrical charge. The alcohol vapor condenses on these air ions. Thus, the ions formed by the passage of a radioactive particle mark the path of the particle. In the cloud chamber, the trail of Alpha particles is straight and wide.
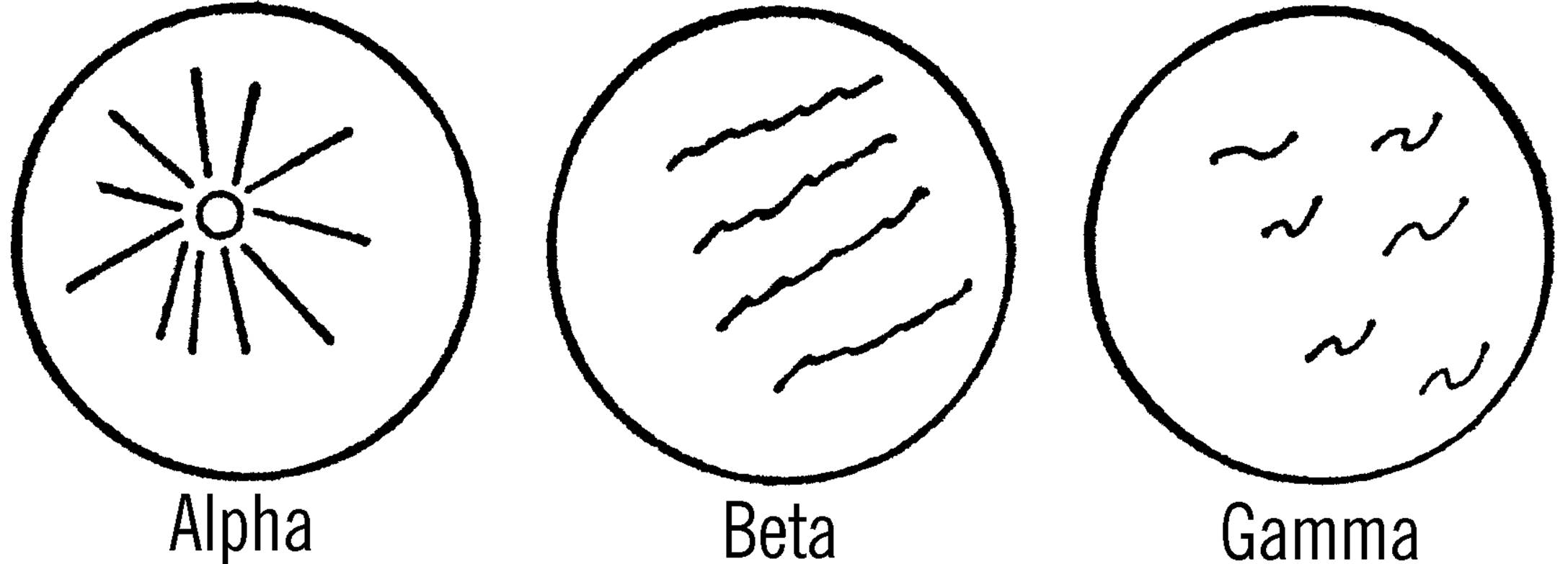
Beta particles are electrons given off as neutrons change to protons. These particles are negatively charged and travel at almost the speed of light. The Beta particles leave curly, wispy trails that are much harder to detect than the trail of Alpha particles.
Gamma rays have a great penetrating power and travel at the speed of light. They will appear occasionally as a spot with trails going in all directions.
Can You Work Like a Scientist?
1. Is copper a better conductor of heat than glass? How can you prove this?
2. Why do you need a band around the top of the gallon jug?
3. What is the difference in temperature between the top and the bottom of the glass chamber? Can you measure this?
4. Does heat increase the rate of evaporation for both alcohol and water?
5. Will the cloud chamber work without using the electronic clearing field?
6. Will the cloud chamber work if you use just plain ice instead of dry ice?
7. Will the chamber work as well if you reverse the leads on the battery?
8. Do you have to clear the field by connecting up the battery every time you want to view the path of particles? What happens if you leave the battery connected up? Are the ions that are formed immediately attracted to the metal plates?
9. If you used a water vapor instead of alcohol vapor, would the chamber work as well?
10.Can you keep water vapor from condensing by removing the dust and ion particles?
11.Which form of radiation is the most common?
12.Can you explain why the tracks of the various particles and rays differ?
13.Will any of the radioactive materials go through paper? You might place a paper barrier in the chamber.
14.Does radiation travel in a straight line? Can you prove this?
15.Can you take pictures of these vapor trails?
16.Remove the radioactive source from the chamber. Observe carefully. Do you still see an occasional vapor trail? This could be a cosmic ray.
17.Can you tell from your chamber what form of radiation is a cosmic ray?
18.Can you shield your chamber so that cosmic rays cannot enter?
19.If some forms of radiation are electrically charged particles, can you change the path of the particles by using a strong magnetic field?
20.Can you devise a simpler chamber than the one described?
21.Are we bothered by cosmic rays more in the daytime or at night?
22.What effect do sunspots have on the amount of cosmic rays?
23.Does radiation help discharge an electroscope? Can you explain the reason?
24.Does rain help bring radiation down from the atmosphere?
25.Another type of cloud chamber is the expansion type. The theory behind it is this:
If air is pumped into a chamber containing alcohol, the temperature is increased as the air is compressed. As the temperature increases, the atmosphere is able to hold more and more vapor. Finally, if the air pressure is released suddenly, the alcohol vapor and air atmosphere expand rapidly. This rapid expansion cools the atmosphere in the chamber. The atmosphere can no longer hold the alcohol vapor at this decreased temperature, so the alcohol condenses on any available nuclei.
Can you design an expansion type of cloud chamber? You might get an idea by examining the chest cavity mentioned in the biology section.11
1 In this project, originally titled “Atomic Time Clock,” you will build a model that simulates, by way of analogy, how radiometric dating can reveal the age of a sample. Radiometric dating (e.g., carbon dating or lead-lead dating) works by comparing the relative abundance of a radioactive isotope to its decay products. This is almost the inverse of a modern “atomic clock,” which is an extremely accurate timekeeping apparatus that relies upon the consistent properties of stable (non-radioactive) atoms.
2 Note that the actual apparatus is that of the “Water Hourglass”.
3 Or she!
4 See Note 28 in Appendix E about radioactive sources, including radioactive paint found on some old alarm clocks.
5 Not all alarm clocks have radioactive paint; see the previous note.
6 Does it need to be genuine shellac? What other materials would work?
7 Model airplane (plastic) cement, Duco cement, or five-minute epoxy.
8 Read Note 29 in Appendix E for an extended discussion about materials for this project.
9 A soldering iron with at least 75 W power is recommended.
10 Slide projectors can often be found used at garage sales and thrift stores. Modern computer projectors will also work well.
11 Or possibly from the “Cloud Jar”.