GED Test Science Flash Review (2015)
PHYSICAL SCIENCE
Which of the following is NOT a physical property?
A. flammability
B. hardness
C. solubility
D. density
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The smallest unit of matter is
A. a compound
B. an atom
C. a molecule
D. a proton
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Which is an example of phase change?
A. oil floating in water
B. oxygen diffusing in water
C. paper burning
D. water freezing
Choice A, flammability is not a physical property. A physical property is something that is observable and measurable and does not involve a chemical reaction. Flammability is a measure of a substance’s ability to burn, a property that cannot be determined without chemically altering the substance. For hardness, solubility, and density, each property is observable and measurable.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Choice B, an atom, is the smallest unit of matter and is composed of neutrons, protons, and electrons. A compound is made of more than one element. A molecule is made of more than one atom. A proton is part of an atom and not a complete unit of matter.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Choice D, water freezing, is a phase change from liquid to solid. Oil floating in water shows a difference in density, not a phase change. When oxygen diffuses in water, it stays as a gas in the water and does not change states. There is no change in state when paper burns. This is an example of a chemical reaction, not a phase change.
Balance the following equation by inserting the correct numbers in the blanks.
____ SnO2 + ____ H2 → ____ Sn + ____ H2O
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Balance the following equation by inserting the correct numbers in the blanks.
____ KOH + ____ H3PO4 → ____ K3PO4 + ____ H2O
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Balance the following equation by inserting the correct numbers in the blanks.
____ NH3 + ____O2 → ____ NO + ____ H2O
1 SnO2 + 2 H2 → 1 Sn + 2 H2O
This equation is balanced because there are four atoms of hydrogen (H) on the left and the right of the equation. There are two atoms of oxygen (O) on each side. There is one atom of Sn on each side.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
3 KOH + 1 H3PO4 → 1 K3PO4 + 3 H2O
This equation is balanced because there are three atoms of potassium (K),
seven atoms of oxygen (O), six atoms of hydrogen (H), and one atom of phosphorus (P) on each side of the equation.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
4 NH3 + 5 O2 → 4 NO + 6 H2O
This equation is balanced because there are four atoms of nitrogen (N), 12 atoms of hydrogen (H), and 10 atoms of oxygen on each side of the equation.
Balance the following equation by inserting the correct numbers in the blanks.
____ Fe + ____ Cl2 = ____ FeCl3
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
All chemical reactions must conserve 1. ____, 2. ____, and 3. ____.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
If heat is released, the reaction is
A. endothermic.
B. exothermic.
C. equivalent.
E. evaporative.
2 Fe + 3 Cl2 = 2 FeCl3
This equation is balanced because there are 2 iron (Fe) atoms, and 6 chlorine (Cl) atoms on each side of the equation.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
All chemical reactions must conserve 1. matter or mass, 2. energy, and 3. electric charge. Matter can neither be created nor destroyed. The same rule applies for energy and overall charge.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice B, exothermic. Endothermic reactions absorb energy. Equivalent refers to balancing equations, not the heat of a chemical reaction. Evaporate refers to part of the hydrologic cycle, not the heat of a chemical reaction.
What conclusion can be drawn from the chart and caption shown here?
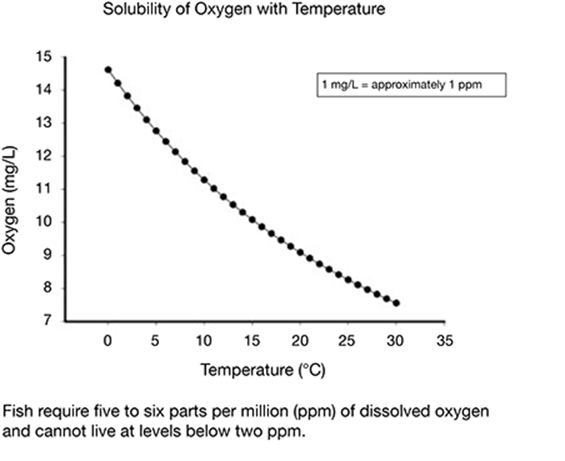
A. Warmer water holds less oxygen.
B. Fish cannot survive in water at 25°C.
C. As temperature increases, oxygen dissolves more quickly.
D. By doubling water temperature, the amount of dissolved oxygen is decreased by half.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice A. The graph shows that the amount of oxygen dissolved in water decreases as water temperature increases.
The text states that fish can live at two ppm and the graph does not indicate any level lower than seven ppm. The graph does not show the rate at which oxygen dissolves. The graph does not show the relationship of water temperature and dissolving oxygen.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
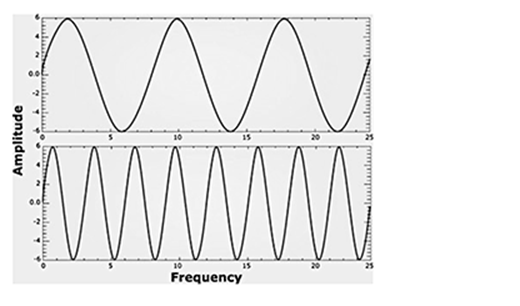
The following image represents sound waves. Label each diagram as low or high frequency.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Calculate the momentum of a 0.25 kg ball that is moving toward home plate at a velocity of 40 m/s.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Calculate the momentum of a 0.20 kg ball that is moving toward home plate at a velocity of 40 m/s.
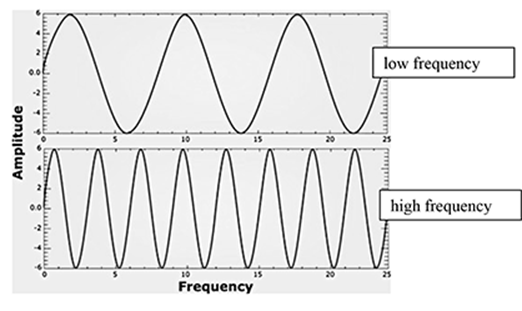
Frequency as it relates to waves is defined as the number of wave cycles that pass in a certain period of time. In sound waves, higher-pitched sounds have a higher frequency. This means that more cycles of waves are compressed into the same period of time. Lower-pitched sounds have lower frequency.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
p = m · v
0.25 kg · 40 m/s = 10 kg · m/s
The formula for momentum is mass multiplied by velocity [p = m · v]. The mass of the ball is 0.25 kg times the velocity of 40 m/s.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
p = m · v
0.20 kg · 40 m/s = 8 kg · m/s
The formula for momentum is mass multiplied by velocity [p = m · v]. The mass of the ball is 0.20 kg times the velocity of 40 m/s.
Calculate the momentum of a 0.15 kg ball that is moving toward home plate at a velocity of 40 m/s.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
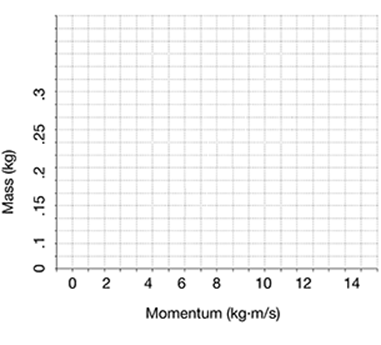
Plot the answers to the next problem on a graph, to represent the relationship between mass and momentum for objects traveling at the same velocity.
Plot the momentum of a 0.25 kg ball, a 0.20 kg ball, and a 0.15 kg ball that are all moving toward home plate at a velocity of 40 m/s.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
p = m · v
0.15 kg · 40 m/s = 6 kg · m/s
The formula for momentum is mass multiplied by velocity [p = m · v]. The mass of the ball is 0.15 kg times the velocity of 40 m/s.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
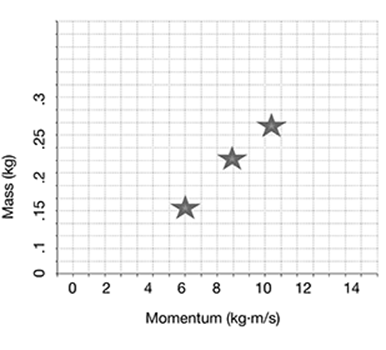
As you can see from the graph, as the mass of the ball increases, the momentum also increases.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
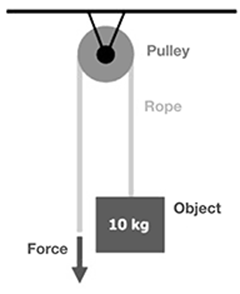
What force is needed to lift the 10 kg object with one fixed pulley (assume there is no mechanical advantage)?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
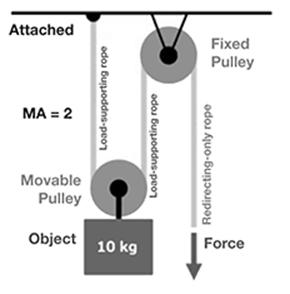
What force is needed to lift the 10 kg object with a two-pulley system (assume there is mechanical advantage)?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is 10 N of force would be needed to raise the 10 kg object since there is no mechanical advantage. A fixed pulley can make it seem easier to lift an object, but the same amount of force is needed to lift an object with a fixed single pulley as without the pulley (no mechanical advantage).
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Double pulley system: If you wanted to lift a 10 kg object 10 cm, you would need to apply five N of force, reducing the force needed by half. A fixed pulley can make it seem easier to lift an object, but the same amount of force is needed to lift an object with a fixed single pulley as it does without the pulley (no mechanical advantage). Certain types of pulleys do produce a mechanical advantage. The more pulleys that are added to the system, the less force is needed to move the object. The mechanical advantage is increased because the force is distributed over the length of the pulley ropes.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
If a force of 20 N is applied to a 2 kg object, the object will accelerate at _______ m/s/s.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
If a force of 40 N is applied to a 2 kg object, the object will accelerate at _______ m/s/s.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A school bus is 20 m long and has a mass of 10,000 kg (10 metric tons) when empty. Determine the weight of the bus in Newtons.
weight = mass × acceleration due to gravity (g)
g = 9.8 m/s2
The correct answer is 10 m/s/s. To find acceleration, you divide force by the mass. 20 N/2 kg = 10 m/s/s.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is 20 m/s/s. To find acceleration, you divide force by the mass. 40 N/2 kg = 20 m/s/s.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The answer is 98,000 N. You calculate the answer by: 10,000 kg × 9.8 m/s2 = 392,000 N.
When you add 84 passengers (high school students) to the 10,000 kg bus, it can add up to 6,400 kg in additional weight.
Determine the weight of the bus in Newtons.
weight = mass × acceleration due to gravity (g)
g = 9.8 m/s2
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A blue whale is 30 m long and has a mass of 40,000 kg (400 metric tons).
Calculate the weight of a blue whale in Newtons
weight = mass × acceleration due to gravity (g)
g = 9.8 m/s2
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Acceleration is force divided by mass.
acceleration = force/mass
If a force of 10 N is applied to a 2 kg object, the object will accelerate at _______ m/s2.
The answer is 160,720 N. You calculate the answer by 16,400 kg × 9.8 m/s2 = 160,720 N.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is 392,000 N. 40,000 kg × 9.8 m/s2 = 392,000 N.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is 5 m/s2. 10N ÷ 2 kg = 5 m/s2.
How much force (in Newtons) is needed to raise the object in the pulley system 4?
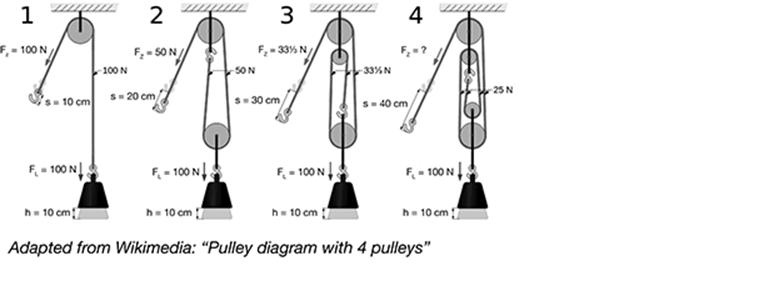
A. 25 N
B. 33 N
C. 50 N
D. 10 N
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The equation for photosynthesis is:
6CO2 + 6H2O + Energy → C6H12O6 + 6O2
Which of the following correctly identify(ies) the reactants in the equation? Choose one or more.
• glucose
• oxygen
• carbon dioxide
• water
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice A, 25N. The diagrams show the mechanical advantage of using multiple pulleys. There is a proportional relationship between the number of pulleys and the force needed to move an object of constant weight. The force acting on the object is 100 N. With one pulley, there is no mechanical advantage and 100 N of force is required to lift the object. In the second system, there are two pulleys and the force is distributed over the two ropes, so the force needed to lift the object is 50 N. With three pulleys, the force needed is divided by one-third (33.3 N). Finally, with four pulleys the force needed to move the object is one-fourth, or 25 N.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
carbon dioxide, water
Carbon dioxide combines with water in the presence of energy. These reactants produce glucose in addition to oxygen as a waste material.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Which of the following is NOT true?
A. Atoms can be destroyed in chemical reactions.
B. Matter is composed of atoms.
C. All atoms of any given element are identical.
D. Atoms of different elements have different properties.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Which of the following statements best describes why a ball eventually slows down after you kick it?
A. An object at rest tends to stay at rest unless a force acts on it.
B. An object in motion tends to stay in motion unless a force acts on it.
C. The amount of momentum an object has depends on its mass and velocity.
D. The strength of the gravitational force depends on the mass of the object.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Acceleration that is negative is called __________________.
Choice A is not true. It is the only false statement. Atoms cannot be destroyed in chemical reactions. They can be rearranged and changed, but cannot be created or destroyed.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice B. The force of friction is acting on the ball to slow it down. An object that is moving continues to move at the same speed in the same direction, unless some force is applied to it to slow it down, to speed it up, or to change its direction. While the other answers are true, they do not explain why the ball slows down.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
An acceleration that is negative (due to an ending velocity that is less than the starting velocity) is called a deceleration. For the velocity of motion to change, the speed and/or the direction must change and a net or unbalanced force must be applied. The amount of acceleration or deceleration is directly proportional to the force applied.
This is an image of a section of a roller coaster. What kind of energy is represented at the top of the roller coaster (W)?
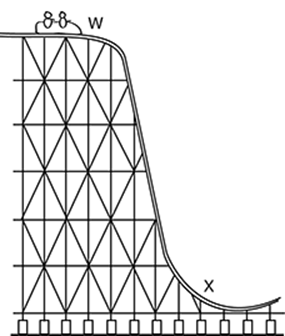
A. kinetic
B. mechanical
C. potential
D. leverage
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The top of the roller coaster represents high potential energy, choice C.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
This is an image of a section of a roller coaster. What kind of energy is represented at the bottom of the roller coaster (X)?

A. kinetic
B. mechanical
C. potential
D. leverage
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Which of the following is NOT a phase change?
A. wood burning
B. water freezing
C. ice melting
D. water condensing
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
This point (X) shows the point at which the coaster has high energy of movement (choice A, kinetic energy).
The bottom of the roller coaster represents high kinetic energy, choice C.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice A, wood burning.
A phase change is a physical process without chemical bonds being formed or broken. In the case of wood burning, chemical bonds are being broken.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Fill in the blanks to describe potential and kinetic energy.
_______ energy is energy that is stored. _______ energy is the energy associated with motion.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The graph shows the solubility of different substances as a function of temperature. What conclusion is NOT true based on the information in the graph?
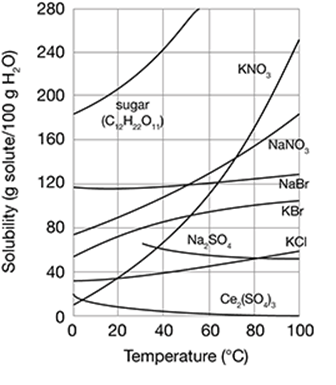
A. The solubility of some substances decreases as water temperature increases.
B. The solubility of sodium bromide (NaBr) is changed very little by increasing the temperature.
C. If you want more sodium nitrate (NaNO3) to dissolve in water, increase the temperature.
D. Increasing the temperature of water will not increase the solubility of sugar (C12H22O11) in water.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Potential energy is energy that is stored. Kinetic energy is the energy associated with motion.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Choice D is not true. In the graph, sugar (C12H22O11) becomes more soluble in water as the temperature is increased. The other options are all true based on the information in the graph.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The graph shows the density of freshwater as it relates to water temperature.
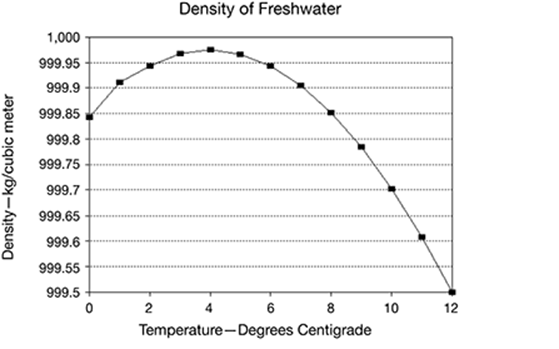
What can you conclude about the density of water from the graph?
A. The density of water increases as temperature increases.
B. The density of water is lowest at the highest temperatures.
C. The density of water decreases as temperature increases.
D. The density of water is highest at the lowest temperatures.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice B. The density of water is lowest at the highest temperatures. The graph shows the density is lowest at 12 degrees centigrade.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
While hiking in a deep canyon, a lost hiker yells out loud. He hears the echo 0.86 seconds after the yell. The speed of the sound wave in the air is 342 m/s. Calculate the distance to the nearest canyon wall.
Here are two formulas that will help.
Velocity = distance/time
Distance = velocity · time
HINT: Remember, the echo represents sound traveling to the wall and back.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Classify each substance as an element, compound, or mixture.
Water (H2O): ___________
Hydrogen (H): ___________
Salt (NaCl): ___________
Glucose (C6H12O6): ___________
Oxygen (O2): ___________
Saltwater: ___________
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Fill in the blanks in the following sentence using the choices that follow.
Within a system, energy can be ______, but can neither be ______ nor ______.
• created
• transformed
• destroyed
147.06 m
The velocity of the sound is 342 m/s. The time it takes to hear the sound (the time to travel to the wall and back) is 0.86 s.
v = 342 m/s, t = 0.86 s (2-way)
If it takes 0.86 seconds to travel to the canyon wall and back, then it takes 0.43 seconds to travel to the wall.
Now use d = v · t
d = v · t = (342 m/s) · (0.43 s) = 147.06 m
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Water (H2O): compound
Hydrogen (H): element
Salt (NaCl): compound
Glucose (C6H12O6): compound
Oxygen (O2): element
Saltwater: mixture
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Within a system, energy can be transformed, but can neither be created nor destroyed.
Balance the following equation.
____ C6H12O6 + ____ O2 = ____ CO2 + ____ H2O
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Write the formula for glucose, which contains six carbon atoms (C), 12 hydrogen atoms (H), and six oxygen atoms (O).
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
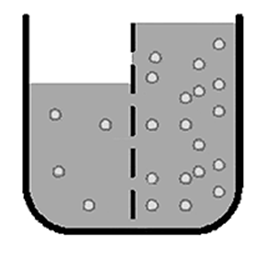
The following illustration shows a solute in a container with a semipermeable membrane.
In which direction the water will move.
1 (C6H12O6) + 6 (O2) = 6 (CO2) + 6 (H2O)
This equation is balanced because there are 18 atoms of oxygen on the left of the equation and on the right. There are 6 carbon (C) atoms on each side of the equation. There are 12 hydrogen (H) atoms on each side of the equation.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
C6H12O6
Other variations in the order of the formula are allowable, such as C6O6H12.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
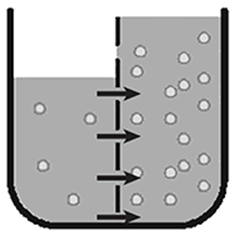
To the right.
The water will move from the area of low solute concentration (in the left section of the diagram) to the area of higher solute concentration (right side of the diagram) until equilibrium is reached.
The movement of water and other types of molecules across membranes (including cell membranes) is important to many life functions in living organisms. Movement of these molecules occurs by diffusion through the membranes, which are semipermeable. These processes, including diffusion and osmosis (the diffusion of water), are sometimes called passive transport since they do not require any energy to occur. The molecules move across the membrane because of osmotic pressure. Osmotic pressure forces highly concentrated molecules to move across a membrane into areas of lower concentration until a balance is reached. This balance is called equilibrium.
Newton’s second law of motion is the Law of Acceleration. The calculation can be stated as shown here:
acceleration = force/mass
If a force of 20 N is applied to a 5 kg object, the object will accelerate at _______ m/s/s.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Label these parts of the diagram with Maximum Potential Energy and Maximum Kinetic Energy.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Which of the following statements about atoms is NOT true?
A. Matter is composed of atoms.
B. Atoms are created and destroyed in chemical reactions.
C. All atoms of a given element are identical.
D. A given compound always has the same relative number and kind of atoms.
4 m/s/s
20 N/5 kg = 4 m/s/s
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
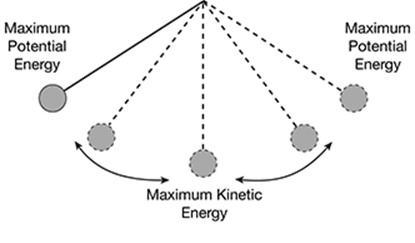
Potential energy is the stored energy, while kinetic energy is associated with motion.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Choice B is not true. Atoms are not created and destroyed in chemical reactions.
Match the part of the atom with the correct definition.
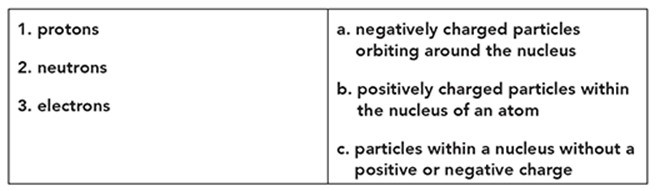
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
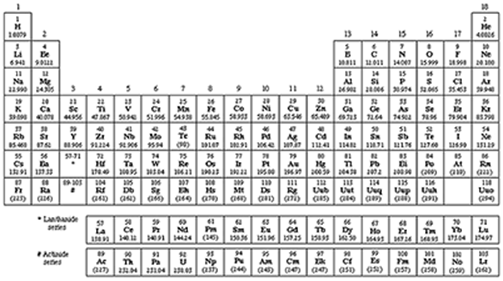
The atomic number of an element is related to the number of protons in the nucleus. Looking at the chart, what can you conclude about the way the elements are organized?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is the difference between ionic and covalent bonds in molecules?
protons—B. positively charged particles within the nucleus of an atom
neutrons—C. particles within a nucleus without a positive or negative charge
electrons—A. negatively charged particles orbiting around the nucleus
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Elements are organized in order of their proton number, atomic number, and mass.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Ionic bonds form when an atom donates one or more electrons to another. Covalent bonds form when electrons are shared between atoms.
Place each of the following in its correct state of matter:
• water
• ice
• steam

. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Place each of the following in its correct state of matter at room temperature:
• diamonds
• perfume
• helium
• vinegar
• metal
• air

. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Which of the following is NOT a change of state?
A. dew forming
B. gold liquefying
C. boiling water
D. blending fruit

. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .

. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Choice D, blending fruit, is not a change of state because blended fruit is composed of both solid and liquid compounds. In this case, blending is the chopping of an object until its solid form becomes something similar to liquid.
What is the measure of how much matter is in an object?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
How is density described?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Assign the following terms to the correct definition:

This is described as mass.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
This is the mass per unit of volume.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
volume—E. how much space is occupied by a substance
elasticity—C. the ability of a substance to return to its original shape after deforming force is applied
solubility—A. the measure of how much a substance will dissolve in another substance
hardness—B. the measure of how resistant a substance is to shape change when a force is applied
viscosity—D. the measure of a substance’s resistance to flow
What do mass, elasticity, solubility, and viscosity all have in common?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Which of the following is a physical property?
A. heat of combustion
B. flammability
C. reactivity
D. boiling point
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is the following reaction an example of?
2 CO + O2 → 2 CO2
A. synthesis
B. decomposition
C. single replacement
D. double replacement
They are all physical properties.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Choice D, boiling point is the only physical property. The others are all chemical properties.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
This is an example of choice A, a synthesis reaction.
What is the following reaction an example of?
CaCO3 → CaO + CO2
A. synthesis
B. decomposition
C. single replacement
D. double replacement
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is the following reaction an example of?
Zn + 2 HCl → ZnCl2 + H2
A. synthesis
B. decomposition
C. single replacement
D. double replacement
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is the following reaction an example of?
AgNO3 + NaCl → AgCl + NaNO3
A. synthesis
B. decomposition
C. single replacement
D. double replacement
This is an example of choice B, a decomposition reaction.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
This is an example of choice C, a single replacement or single displacement reaction.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
This is an example of choice D, a double replacement reaction.
What do all chemical reactions conserve? List at least two.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is the difference between an endothermic reaction and an exothermic reaction?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Ice melting is an example of which type of reaction?
• endothermic
• exothermic
All chemical reactions conserve matter (mass), energy, and electrical charge.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
An endothermic reaction absorbs heat, whereas an exothermic reaction releases heat.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
This is an example of an endothermic reaction, as the ice is absorbing heat from the surrounding area in order to change its state from a solid to a liquid.
Burning propane is an example of which type of reaction?
• endothermic
• exothermic
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Cooking an egg is an example of which type of reaction?
• endothermic
• exothermic
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Condensation is an example of which type of reaction?
• endothermic
• exothermic
This is an example of an exothermic reaction, as the burning propane releases heat into the surrounding area in order to change its state from a liquid to a gas.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
This is an example of an endothermic reaction, as the egg is absorbing heat in order to change its state from a liquid to a solid.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
This is an example of an exothermic reaction as the water in gas form is releasing heat in order to change its state from a gas to a liquid.
What is the difference between a homogeneous mixture and a heterogeneous mixture?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Is seawater a homogeneous or heterogeneous mixture?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Is smoke a homogeneous or heterogeneous mixture?
A homogeneous mixture has the same composition throughout and the components can’t be visually separated. A heterogeneous mixture has variation in its composition throughout and the components can sometimes be visually separated.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Seawater is a homogeneous mixture. While it is composed of several elements, it is the same composition throughout and cannot be visually separated into its components.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Smoke is a heterogeneous mixture, as it is composed of solids and gas in the form of carbon particles and air.
A uniform mixture is called a ___________.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
___________ is reached when the solution cannot hold additional solute.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
When playing pool, a cue ball is hit at a stationary eight ball. The cue ball has energy and when it hits the eight ball transfers that energy over. The cue ball then slows down. What is this an example of?
A uniform mixture is called a solution.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Saturation is reached when the solution cannot hold additional solute.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
This is an example of the conservation of energy.
When sunlight hits a plant and that plant takes the sunlight and uses photosynthesis to grow, that is an example of ____________ of energy.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What type of energy is exhibited by someone jumping rope?
• kinetic
• potential
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What type of energy is exhibited by a child at the top of a slide?
• kinetic
• potential
When sunlight hits a plant and that plant takes the sunlight and uses photosynthesis to grow, that is an example of transformation of energy.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
This is kinetic energy.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
This is potential energy.
What type of energy is exhibited by a fish swimming?
• kinetic
• potential
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What type of energy is exhibited by someone holding a yo-yo?
• kinetic
• potential
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Which of the statements about conduction is NOT true?
• Wood is a great conductor.
• Metal is an excellent conductor.
• A bad conductor is known as an insulator.
This is kinetic energy.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
This is potential energy.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Wood is not a great conductor of heat and is instead actually an insulator. This is why we use wooden spoons in boiling pots of water.
What is the word for the reflection of sound waves?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Which of these statements about waves is true?
A. A ray of light hits a water surface and is both reflected and refracted.
B. Radio waves have the shortest wavelengths.
C. Long wavelengths carry more energy.
D. Electromagnetic waves do not include visible light.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
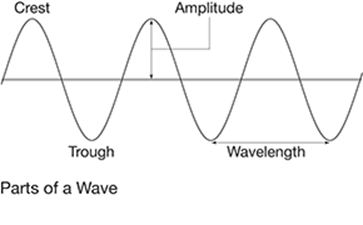
Are the parts of the wave in this diagram labeled correctly?
echoes
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice A. A ray of light hits a water surface and is both reflected and refracted.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
No. Amplitude measures height of the crest. The crest is the top of the wave, whereas the trough is the bottom of the wave. Wavelength measures the length of a full wave before it repeats. It’s easiest to measure it from trough to trough or crest to crest.

In sound waves, sounds with a higher pitch have a higher ___________.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The table shows the maximum amount of oxygen gas that can be dissolved in water at various temperatures.
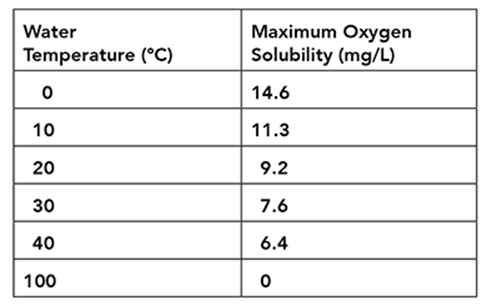
The data in the table support which of the following statements about the relationship between water temperature and oxygen solubility?
A. Bodies of water with a lower average temperature can support a higher concentration of dissolved oxygen.
B. Bodies of water with an average temperature higher than 40°C contain no dissolved oxygen.
C. A 10°C increase in water temperature results in an approximately three mg/L change in oxygen solubility.
D. The oxygen solubility of a body of water is affected by many variables, including water temperature.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
In sound waves, sounds with a higher pitch have a higher frequency. This means that more waves are compressed into a period of time.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice A. The table’s data shows the relationship between water temperature and oxygen solubility demonstrating that with water temperature increases, maximum oxygen solubility decreases. This shows that water temperature and dissolved oxygen concentration have an inverse relationship, with highest dissolved oxygen concentrations occurring at the lowest temperatures.
According to the table, bodies of water with an average temperature of 40°C have a maximum oxygen solubility of 6.4 mg/L, and bodies of water with an average temperature of 100°C contain no dissolved oxygen. Temperatures between these two should support oxygen concentrations between 6.4 and 0 mg/L. An increase from 0°C to 10°C results in a 3.3 mg/L increase in oxygen solubility, however, oxygen solubility does not continue to increase by the same increment with each additional 10°C increase in temperature. Also, though many variables can affect oxygen solubility, the table focuses only on the relationship between oxygen solubility and water temperature.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The table shows the maximum amount of oxygen gas that can be dissolved in water at various temperatures.
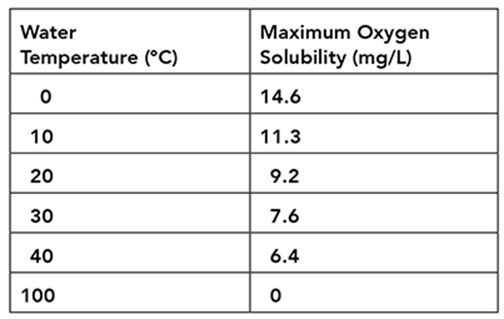
Researchers find that a body of freshwater with an average temperature of 21°C has a dissolved oxygen concentration of 7.2 mg/L. What is a reasonable prediction of the water’s dissolved oxygen concentration after the population size of freshwater grasses doubles?
A. 6.3 mg/L
B. 7.2 mg/L
C. 8.5 mg/L
D. 14.4 mg/L
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A marathon runner consumes foods with a high carbohydrate content before and during a race to prevent muscle fatigue. This practice, called carb loading, supports which of the following energy transformations within the runner’s body?
A. chemical to thermal
B. thermal to kinetic
C. kinetic to thermal
D. chemical to kinetic
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice C. Grasses release oxygen into the environment as a by-product of photosynthesis. Using this reasoning, it can be predicted that an increase in freshwater grasses will increase the dissolved oxygen concentration. Based on the data in the table, an increase to 8.5 mg/L brings the dissolved oxygen concentration closer to the maximum oxygen solubility for a body of water with an average temperature of 21°C.
Aquatic plants like freshwater grasses release oxygen into the environment. A dissolved oxygen concentration of 6.3 mg/L would result from an event that decreases the amount of dissolved oxygen in the water. A dissolved oxygen concentration of 7.2 mg/L would indicate no change in the ecosystem. A change in the freshwater grass population would alter the amount of dissolved oxygen in the water. A doubling of the freshwater grass population would cause an increase in dissolved oxygen concentration, but not a doubling. According to the table, a dissolved oxygen concentration of 14.4 mg/L far exceeds the maximum oxygen solubility for a body of water with an average temperature of 21°C.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice D. The runner takes in chemical energy in the form of carbohydrates. This chemical energy is transformed into kinetic energy as the runner’s muscles contract and relax, causing the runner to move. Runners carb load to ensure that their body has enough chemical energy to be transformed into the kinetic energy required to run a marathon.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A highway patrol officer is monitoring the speed of vehicles along a stretch of highway with a speed limit of 55 mph. The results are shown here.
Vehicle 1: 61 mph
Vehicle 2: 48 mph
Vehicle 3: 61 mph
Vehicle 4: 51 mph
Vehicle 5: 59 mph
What is the average speed of the five vehicles? (You may use a calculator to answer this question.)
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The average speed can be determined by adding the individual vehicle speeds and dividing by the total number of vehicles. This is calculated as![]() = 56 mph.
= 56 mph.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Matter exists in solid, liquid, and gas states. A substance may change between these three states. State changes can alter the physical properties of a substance, as depicted in the following models.
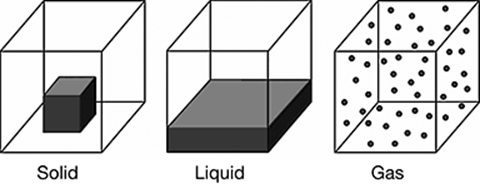
Which summary best explains the model of the states of matter?
A. Liquids have a fixed shape like solids, but assume the volume of the container like gases.
B. Liquids have a fixed volume and shape, like solids. Gases assume the volume and shape of the container.
C. Liquids have a fixed volume like solids, but assume the shape of the container like gases.
D. Liquids assume the volume and shape of the container, like gases. Solids have a fixed volume and shape.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice C. As shown in the model, a solid has a fixed volume and shape. A liquid has a fixed volume, but assumes the shape of the container. A gas assumes the volume and shape of the container. A liquid has one property in common with solids, and one property in common with gases.
The first summary reverses the properties of liquids. The second falsely states that liquids have fixed shapes. The fourth falsely states that liquids assume the volume of the container.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .

Based on the model, which state change increases the density of a substance?
A. gas to liquid
B. solid to gas
C. liquid to gas
D. solid to liquid
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The term exothermic describes a process in which energy is released, usually as thermal energy. The term endothermic describes a process in which thermal energy is absorbed.
Which of the following is an example of an exothermic process?
A. a candle burning
B. a snowbank melting
C. a loaf of bread baking
D. a plant making sugar
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice A, gas to liquid. The density of a substance describes how tightly packed the substance’s molecules are. As shown in the model, a substance’s molecules are most spread out when in the gas state. This means that a substance’s density is lowest when in the gas state. The substance’s density increases when going from gas to liquid state because the molecules become more tightly packed.
A substance’s molecules become more spread out when changing from solid to liquid state, liquid to gas state, and solid to gas state. This causes the substance’s density to decrease.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice A, a candle burning. Burning a candle is an exothermic process because thermal energy, or heat, is released as a result of the process.
Melting a snowbank is an endothermic process because the input of heat is required to melt the snow. Baking a loaf of bread is also an endothermic process because the input of heat is required to convert the ingredients to bread. And photosynthesis is an endothermic process because the input of energy (sunlight) is required for plants to make sugar. This means that energy is absorbed during the process, not released.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The graph represents the motion of a remote-controlled car. The car’s acceleration, or change in velocity, is indicated by the slope of the graph.
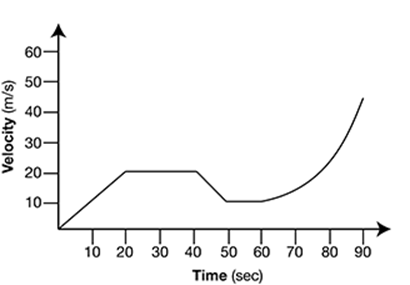
During which time period was the car experiencing a constant positive acceleration?
A. between 0 and 20 seconds
B. between 20 and 40 seconds
C. between 40 and 50 seconds
D. between 50 and 90 seconds
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice A. The car has a constant positive acceleration when the car’s velocity is increasing at a steady, or constant, rate. Between 0 and 20 seconds, the graph moves upward in a straight diagonal line, indicating that the velocity is increasing at a constant rate.
Between 20 and 40 seconds, the car is maintaining a constant velocity of 20 m/s. Since the velocity is constant within this time period, the car is not accelerating (has an acceleration of 0 m/s2). Between 40 and 50 seconds, the car’s velocity is decreasing at a constant rate. This indicates a constant negative acceleration. Between 50 and 90 seconds, the car’s velocity is increasing, but not at a constant rate. The graph moves upward in a curved line within this time period, indicating that the velocity is increasing at a variable rate.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The mechanical advantage (MA) of a machine is a measure of how much the machine multiplies the input force applied to it.
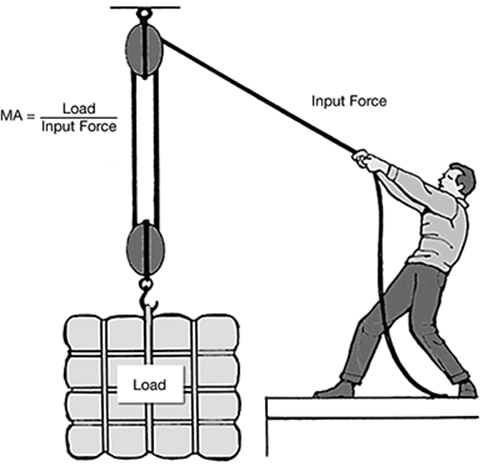
The table shows the input force required to lift different loads using the pulley system shown in the illustration.
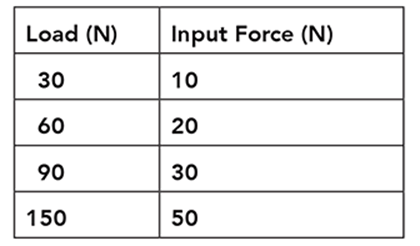
Based on the data in the table, what happens to the mechanical advantage of the pulley system as the load size increases?
A. The mechanical advantage increases at a constant rate.
B. The system’s mechanical advantage does not change.
C. The pulley system multiplies the mechanical advantage.
D. The mechanical advantage decreases at a constant rate.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice B. The mechanical advantage of a pulley system does not change with the load. Mechanical advantage is calculated as load divided by input force. In the data table, dividing each load by its corresponding input force produces a mechanical advantage of three.
As the load size increases, the input force required to lift the load increases at a constant rate. The mechanical advantage of the pulley system does not change. A pulley system multiplies the input force, not the mechanical advantage, applied to a load. No decrease in mechanical advantage occurs with an increase in load. The mechanical advantage of a pulley system is constant regardless of the size of the load.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The mechanical advantage (MA) of a machine is a measure of how much the machine multiplies the input force applied to it.

The table shows the input force required to lift different loads using the pulley system shown in the illustration.
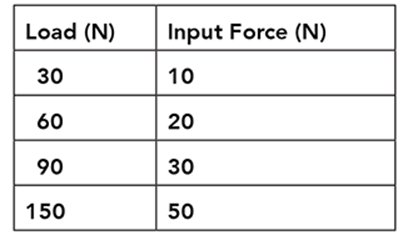
A one Newton load has a mass of 10 grams. According to the table, what is the maximum mass that can be lifted by the pulley system using an input force of 50 Newtons?
A. 15 grams
B. 50 grams
C. 150 grams
D. 1,500 grams
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
According to the table, an input force of choice B, 50 grams can lift a 150 N load. If a 1 N load has a mass of 10 grams, the mass of a load can be determined by multiplying the force of the load by 10. A 150 N load therefore has a mass of 1,500 grams.
Fifteen grams is the result of dividing the force of the load (150 N) by 10. The mass of the load is determined by multiplying, not dividing, the force of the load by 10; 50 grams is the value of the input force, not the mass of the load; 150 grams is the value of the force of the load in Newtons, not the mass of the load in grams.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is a catalyst?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is centripetal force?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is a compound?
An agent that changes the rate of a reaction, without itself being altered by the reaction.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The net force that acts to result in centripetal acceleration. Centripetal force is not an individual force, but the sum of the forces in the radial direction. It is directed toward the center of the circular motion.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A substance composed of more than one element that has a definite composition and distinct physical and chemical properties. Examples include carbon dioxide, sucrose (table sugar), and serotonin (a human
brain chemical).
What is a crystal?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is a decibel?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is density?
A solid in which atoms or molecules have a regular repeated arrangement.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A unit of measure for the relative intensity of sounds.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The mass of a substance for a given unit volume. A common unit of density is grams per milliliter (g/ml).
If a person walks forward one mile in 15 minutes, and then back one mile to the starting point, what measurement would be described as 0 miles?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
How can magnitude be defined?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is a formula for acceleration?
Displacement compares the ending point to the starting point. If a person were to walk forward one mile in 15 minutes, the displacement would be one mile. If a person were to walk one mile forward and one mile back in half an hour, the distance traveled is two miles, but the displacement is zero. Final velocity would be zero and speed would be four miles per hour.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Magnitude can be defined as the measure of a standard unit (for example, 30 miles per hour).
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Velocity divided by elapsed time.
What is a formula for momentum?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is deceleration?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
momentum = mass × velocity
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
This is a state in which acceleration is calculated to be a negative number.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Refer to the following passage to answer the next nine questions.
In the late seventeenth century, Isaac Newton explained light as consisting of particles. But, in the early twentieth century, physicists began explaining light, not as a particle, but rather as a wave. A wave is a periodic oscillation. The shape of a wave starts from a zero level and increases to the highest point or crest. Then, it decreases past zero to its lowest level or trough. From the trough, it rises again to zero. This wave pattern repeats itself over time. The wave has three properties that describe it: amplitude, wavelength, and frequency. Amplitude (A) is the distance from the zero point to the crest of the wave and has the SI unit of meters (m). Wavelength (λ) is the distance from the peak of one wave to the peak of the next wave or the trough of one wave to the trough of the next wave; λ has the SI unit of meters (m). The frequency (ν) is the number of wave cycles per unit of time; the SI unit of frequency is the hertz (Hz). The speed of a wave is the product of the wavelength and the frequency. In the case of a light wave, the speed of light (c) is a constant (3 × 108 m/s) and is described by this formula: c = λν. The wavelengths of light vary from extremely short gamma rays (λ < 10–12 m) to very long radio waves (λ > 1 m).
According to this passage, what is a wave?
A. the distance from one peak to the next
B. the highest point
C. a periodic oscillation
D. the distance from the zero point to the highest point
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
According to this passage, what is a crest?
A. the distance from one peak to the next
B. the highest point
C. a periodic oscillation
D. the distance from the zero point to the highest point
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice C, a periodic oscillation. This can be found in the sentence “A wave is a periodic oscillation.” The word is signals the definition.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice B, the highest point. This can be found in the sentence “The shape of a wave starts from a zero level and increases to the highest point or crest.” The word or signals the definition.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
According to this passage, what is a wavelength?
A. the distance from one peak to the next
B. the highest point
C. a periodic oscillation
D. the distance from the zero point to the highest point
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
According to this passage, what is amplitude?
A. the distance from one peak to the next
B. the highest point
C. a periodic oscillation
D. the distance from the zero point to the highest point
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
According to this passage, what does the symbol λ mean?
A. amplitude
B. wavelength
C. frequency
D. speed of light
The correct answer is choice A, the distance from one peak to the next. This can be found in the sentence “Wavelength (λ) is the distance from the peak of one wave to the peak of the next wave or the trough of one wave to the trough of the next wave.” The word is signals the definition.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice D, the distance from the zero point to the highest point. This can be found in the sentence “Amplitude (A) is the distance from the zero point to the crest of the wave and has the SI unit of meters (m).” The word is signals the definition.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice B, wavelength. This can be seen in the sentence “Wavelength (λ) is the distance from the peak.”
According to this passage, what does the symbol A mean?
A. amplitude
B. wavelength
C. frequency
D. speed of light
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
According to this passage, what does the symbol ν mean?
A. amplitude
B. wavelength
C. frequency
D. speed of light
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
According to this passage, what does the symbol c mean?
A. amplitude
B. wavelength
C. frequency
D. speed of light
The correct answer is choice A, amplitude. This can be seen in the sentence “Amplitude (A) is the distance from the zero point to the crest.”
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice C, frequency. This can be seen in the sentence “The frequency (ν) is the number of wave cycles.”
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice D, speed of light. This can be seen in the phrase “the speed of light (c) is a constant.”
According to this passage, how would you describe the formula c = λν?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
It is in the passage as “The speed of a wave is the product of the wavelength and the frequency.”
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Refer to the following passage to answer the next three questions.
Oxygen is a very corrosive substance and will combine with many other substances or oxidize other substances. A common example is that of a piece of iron left out in the air. Over time, the iron rusts. The rust is a chemical change and can be described by a chemical reaction. Four atoms of solid iron (Fe) combine with three molecules of gaseous oxygen (O2) from the air to form two molecules of solid iron oxide (Fe2O3). The chemical reaction can be written by this chemical equation:
4 Fe (s) + 3 O2 (g) → 2 Fe2O3 (s)
This class of chemical reaction is called a combination reaction.
Using the information presented in the passage, assign each substance’s symbol to its name.

• O2
• Fe2O3
• Fe
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
According to this passage, what does the word oxidize mean?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .

The symbols Fe, O2, and Fe2O3 stand for iron, oxygen, and iron oxide. You can tell that in the parentheses after each substance is mentioned in the fourth sentence, where the chemical reaction is described.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Note the sentence “Oxygen is a very corrosive substance and will combine with many other substances or oxidize other substances.” Oxidize means to combine with oxygen.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
According to this passage, what does the phrase combination reaction mean?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
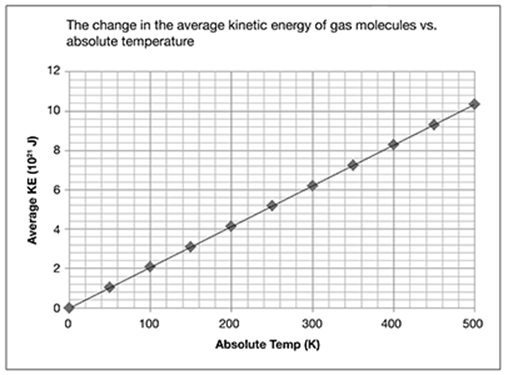
Complete the following statements using the choices that follow.
• In this graph, the numbers on the horizontal (right-to-left), or x-axis, represent the ____________.
• In this graph, the numbers on the vertical (down-to-up), or y-axis, represent the ___________.
• temperature in degrees Kelvin (K)
• average kinetic energy (KE) of gas molecules
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A combination reaction may be one where molecules combine together to form one product. You can get this from the word combine in the fifth sentence, where the chemical reaction is described.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
In this graph, the numbers on the horizontal (right-to-left), or x-axis, represent the temperature in degrees Kelvin (K).
In this graph, the numbers on the vertical (down-to-up), or y-axis, represent the average kinetic energy (KE) of gas molecules.
It’s important to note the axis labels. Look at the vertical or y-axis, for example. You see that the numbers are labeled 2, 4, 6, 8, 10, and 12. But if you look at the axis label, each number represents 1021 joules (J), not whole numbers. This distinction could be very important if you were asked to answer questions based on the graph.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
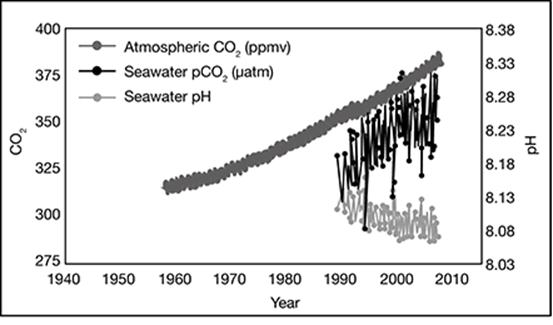
What are the items that are correlated in this graph in your own words?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A look at the vertical axes and the legend tells you that this graph shows the correlation between the changes in carbon dioxide (CO2) levels in the atmosphere and the ocean over time, as well as the pH of seawater over time.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
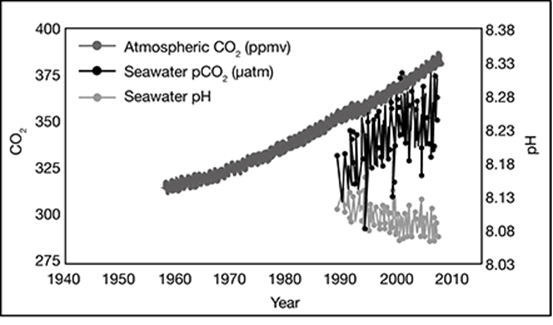
In this graph, which of the following statements is true?
A. As the years passed, atmospheric CO2 decreased, seawater pCO2 remained the same, and seawater pH decreased.
B. As the years passed, atmospheric CO2 increased, seawater pCO2 decreased, and seawater pH remained the same.
C. As the years passed, atmospheric CO2 increased, seawater pCO2 increased, and seawater pH decreased.
D. As the years passed, atmospheric CO2 decreased, seawater pCO2 increased, and seawater pH increased.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice C. The overall levels of the atmospheric CO2 and seawater pCO2 both increased and the seawater pH decreased.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
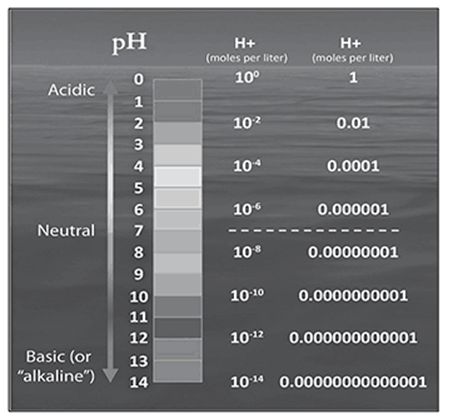
Look at the pH scale. What is its range?
The pH scale in this chart has values from ___ to ___.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The pH scale in this chart has values from 0 to 14.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .

A chemist measures a solution with a pH value of 9.0. What is the hydrogen ion concentration?
A. 10–9 moles per liter
B. 109 moles/liter
C. 1/9 moles per liter
D. 9.0 moles per liter
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice A, 10–9 moles per liter. Note that each number on the pH scale corresponds to a negative exponent when the hydrogen ion concentration is expressed in scientific notation. pH 2.0 = 10–2 moles per liter, pH 4.0 = 10–4 moles per liter, and pH 6.0 = 10–6 moles per liter. So, pH 9.0 = 10–9 moles per liter.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .

If a chemist measures that a solution has a pH value of 5.5, this indicates the solution is __________.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
If a chemist measures that a solution has a pH value of 5.5, this indicates the solution is acidic. Any pH value below 7.0 indicates an acidic substance.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
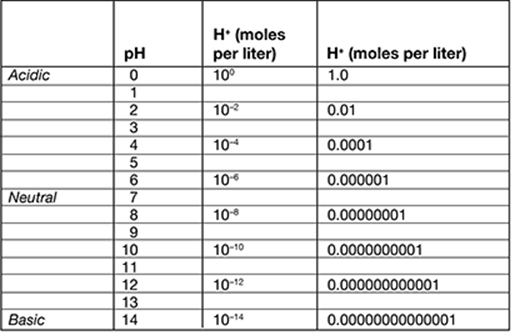
Based on the information in the table, which of these solutions has the greatest concentration of hydrogen ions?
A. pH 2.5
B. pH 4.0
C. pH 7.5
D. pH 10.5
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice A, pH 2.5. Notice that as the pH values increase, the concentration of hydrogen ions decreases (the numbers in the hydrogen ion concentration columns get smaller). So, the lower the pH value, the greater the hydrogen ion concentration. Therefore, pH 2.5 has the highest hydrogen ion concentration of the choices.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Refer to the following passage to answer the next six questions.
A group of students want to know what effect meat tenderizer will have on starches, fats, and proteins.
The group hypothesizes that meat tenderizer will break down proteins, but not starches or fats.
They formulate the following experimental design:
1. Fill six jars with water.
2. Add nine grams of meat tenderizer to three of the jars of water; stir until dissolved.
3. Place one sample of starch, of fat, and of protein in each of the three jars containing meat tenderizer.
4. Place one sample of starch, of fat, and of protein in the three remaining jars.
5. Put lids on all six jars.
6. Observe changes after 24 hours.
What possible sources of errors do you see in this design? Is the hypothesis stated adequately to make a predictable result?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Look at the first step of the experimental design. Is there something not specified?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is unclear about step 2? Is there something not specified?
The wording of your response may vary.
In this case, the hypothesis predicts that the meat tenderizer will break down proteins, but not starches or fats. However, what is exactly meant by “break down” is not specified.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The wording of your response may vary.
Yes, the size of the jars, as well as the amount of water added, is not specified.
Other things to consider: Are the jars glass or plastic? Do six jars comprise a representative sample of the whole population?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The wording of your response may vary.
Although the amount of meat tenderizer is specified, there is no brand or type of meat tenderizer specified. This might be important.
What is unclear about steps 3 and 4? Is there something not specified?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is unclear about step 6? Is there something not specified?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
If you were to recreate this experiment at home, do you think it would produce accurate and precise data?
The wording of your response may vary.
Although the directions say to place one sample of starch, fat, and protein in each jar, the amount and types of samples are not specified. What is the source of the starch: bread flour, cornmeal, etc.? What is the source of the fat: butter, lard, vegetable oil, etc.? What is the source of the protein: ground beef, pork, chicken, fish, soybean, etc.?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The wording of your response may vary.
What type of changes should you look for: changes in size, weight, color, etc.? Under what conditions were the jars kept? Temperature, humidity, and the amount of light are critical variables in many experiments.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The current design is most likely not specific enough to produce accurate data.
Refer to the following passage to answer the next four questions.
A paint company has developed a new brand of outdoor latex paint (brand X) that it thinks might be more durable than another company’s brand (brand Y). To save money, the company has employees paint boards of different scrap wood with brand X and brand Y paints. They paint boards with the same number of coats of paint and measure the paint thickness of each board. They place matched boards painted with brand X and brand Y in different environments (desert, temperate forest, arctic tundra) for 12 months. After 12 months, they measure the paint thickness of each board again. They find that the boards painted with brand Y are thinner than those painted with brand X. They conclude that brand X is more durable than brand Y.
What is the hypothesis of this experiment?
If _____________, then ___________.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Is the hypothesis of this experiment testable?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The wording of your hypothesis may vary.
If brand X is more durable than brand Y under outside conditions, then the paint thickness of boards painted with brand X will be more than brand Y.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Yes. This hypothesis is testable.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
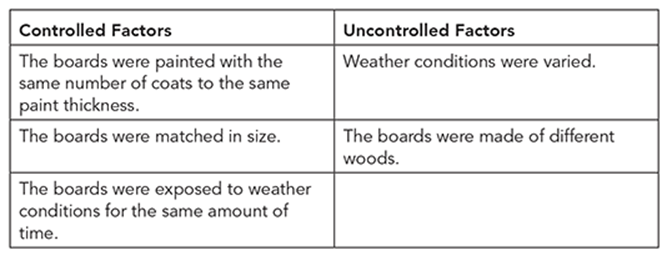
Identify the controlled and uncontrolled factors in the experiment.
Place each of the following factors into its correct place in the table.
• The boards were painted with the same number of coats to the same paint thickness.
• The boards were matched in size.
• Weather conditions were varied.
• The boards were exposed to weather conditions for the same amount of time.
• The boards were made of different woods.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Name three ways this experiment could be improved.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The wording of your response may vary.
1. All the boards could be made of the same type of wood.
2. Rather than paint separate boards, half of each board could be painted with each brand of paint.
3. The environmental conditions could be more rigorously controlled in artificial ovens and freezers.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Refer to the following passage to answer the next three questions.
When applying the brakes in a moving car, the force of friction between the road surface and the vehicle tires is what stops the car. The force of friction depends on the coefficient of friction, which varies with the road conditions (dry, wet, icy) and seasons (summer, winter). In an accident, a police officer can measure the braking distance by the skid marks, note the road conditions, and determine the initial velocity of the car. The data are shown in the graph.
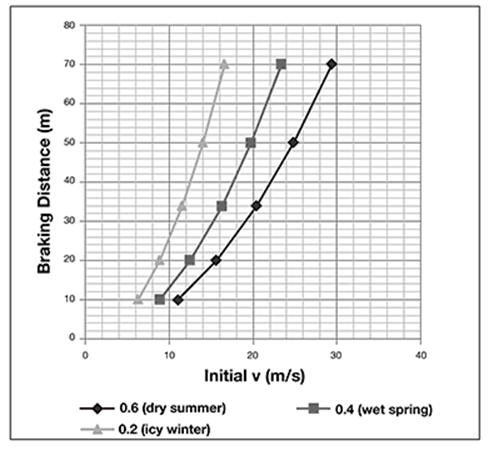
On a road under wet spring conditions, a car has a braking distance of 40 m. The initial speed of the car was ______ m/s.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
On a road under wet spring conditions, a car has a braking distance of 40 m. The initial speed of the car was 18 m/s.
First, use a ruler to trace a horizontal line from 40 m on the y-axis until it meets the curve for wet spring conditions. From that point, trace a perpendicular line to the x-axis and read the initial speed, which is 18 m/s.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
On a road under icy winter conditions, a car is traveling at 10 m/s initially when it brakes. The car will travel _____ m before it stops.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
On a road under wet summer conditions, a car has a braking distance of 60 m. How fast was the car going initially?
A. less than 15 m/s
B. 15–22 m/s
C. 22–27 m/s
D. greater than 27 m/s
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
On a road under icy winter conditions, a car is traveling at 10 m/s initially when it brakes. The car will travel 25 m before it stops.
First, use a ruler to trace a vertical line from 10 m/s on the x-axis until it meets the curve for icy winter conditions. From that point, trace a horizontal line to the y-axis and read the braking distance, which is 25 meters.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice C. There is no curve for wet summer conditions, but these conditions must fall between the curves for wet spring and dry summer. Use a ruler to trace a horizontal line from 60 m on the y-axis until it meets the curve for wet spring conditions. This is the minimum speed. From that point, trace a perpendicular line to the x-axis and read the initial speed, which is 22 m/s. Repeat the process, but continue the horizontal line until it reaches the dry summer curve. This is the maximum speed. From that point, trace a perpendicular line to the x-axis and read the initial speed, which is 27 m/s. So, the car had to be traveling between 22 m/s and 27 m/s.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Refer to the following passage to answer the next four questions.
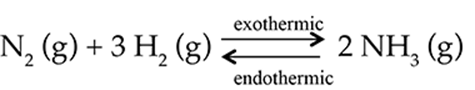
The Haber process is a chemical reaction where nitrogen and hydrogen gases are combined to form ammonia gas. The reaction is represented by this chemical equation:
Chemists studied the effects of increasing pressure on the gases in the reaction. The same amounts of nitrogen and hydrogen gases were combined in a fixed chamber. They increased the pressure of the chamber from 0 to 400 atmospheres (atm). They repeated the experiment at several fixed temperatures from 350°C to 550°C. In each case, they measured the percent yield of ammonia produced in the reaction. The data are shown in the graph:
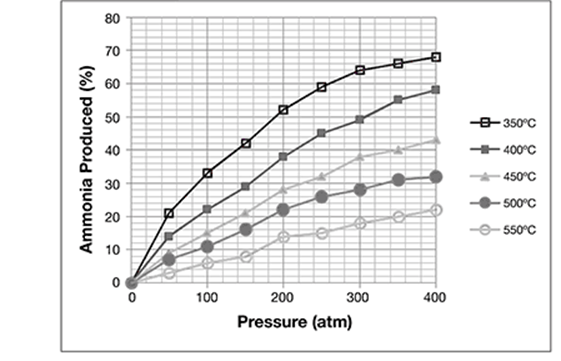
Suppose that you are a chemical engineer and must make a facility that will produce large amounts of ammonia using the Haber process. What would be the best conditions to produce the greatest percent yield of ammonia?
____ atm at ____°C
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
400 atm at 350°C
The best conditions to produce the greatest percent yield of ammonia would be 400 atm at 350°C. These conditions have the highest yield of ammonia (68%).
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Which is the highest pressure at which the reaction can be run while still guaranteeing a less than 55% ammonia yield?
A. 100 atm
B. 200 atm
C. 300 atm
D. 400 atm
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Which is the highest temperature at which the reaction can be run while still guaranteeing a lower than 26% ammonia yield?
A. 350°C
B. 400°C
C. 500°C
D. 550°C
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
When considering ammonia production, what are the best conditions at which to run the Haber reaction?
____ atm at ___°C
The correct answer is choice B, 200 atm.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice D, 550°C.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
400 atm at 350°C
In a chemical reaction, the chemist follows the concentrations of four substances (A–D) over time. The data are shown in the graph.
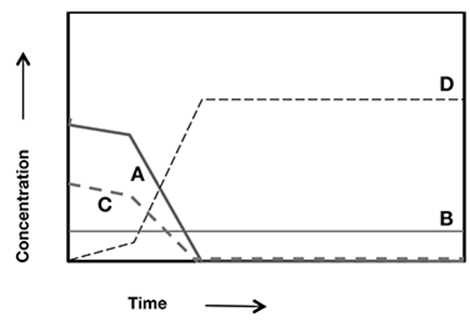
Which chemical reaction is the proper conclusion from the data?
A. A + B → C + D
B. B + D → A + C
C. A + C → D
D. C + D → A
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice C. The concentrations of A and C decrease with time in the same manner, so they are reactants. The concentration of D increases with time, so it is a product.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A student drops a ball from a tall building, while another student videotapes the ball’s path. They repeat the experiment 10 times. From the videotape, they measure the distance and calculate the velocities and accelerations with time. They average the values and plot them on graphs shown here.
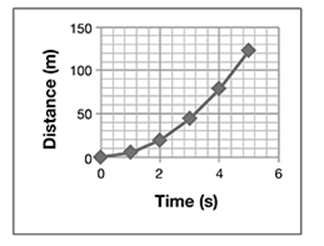
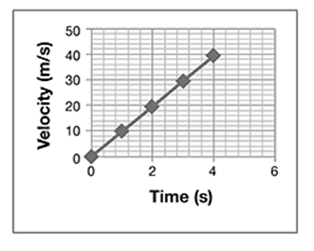
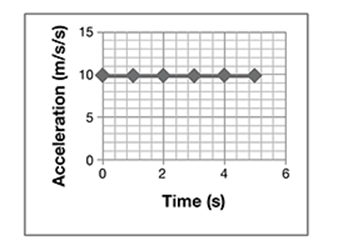
Which conclusions about the ball’s motion are true?
A. The velocity is constant, the acceleration decreases, and the distance increases.
B. Acceleration is constant, while velocity and distance increase.
C. The distance is constant, the velocity decreases, and the acceleration increases.
D. Acceleration is constant, distance increases, but the velocity decreases.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice B. The acceleration of a falling object is constant, while velocity increases linearly, and distance increases with the time squared.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A student drops a ball from a tall building, while another student videotapes the ball’s path. They repeat the experiment 10 times. From the videotape, they measure the distance and calculate the velocities and accelerations with time. They average the values and plot them on graphs.



What is one way to simplify the description of the results of this experiment?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
One way to simplify the presentation might be to eliminate the acceleration graph and verbally state the result:
Acceleration of the ball was constant at 10 m/s/s.

Alternatively, the velocity graph could be described verbally. For example, the velocity of the ball increased linearly from zero at a constant rate of 10 m/s.
Note: The rate of a linear graph is the slope of the line.
Pick any two points on the line and use the slope formula. For example, use (4,40) and (0,0). The slope becomes:
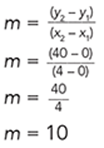
The acceleration of the ball was constant at 10 m/s/s. So, the only graph that needs to be displayed is the graph of distance versus time.

. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
An audit of four preschools in a district shows the number of students and the student-related educational expenses. The data are shown in the table.
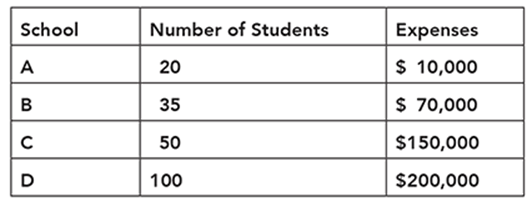
Which school has the greatest expenditure per student?
A. school C
B. school B
C. school A
D. school D
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Which set of data supports the hypothesis that force on a spring (F) increases non-linearly with the displacement from zero (x)?
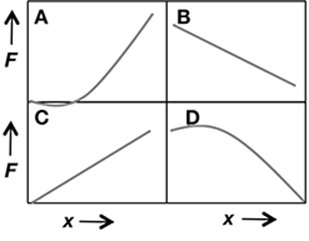
A. set A
B. set B
C. set C
D. set D
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice A. School C spends $3,000 per student, which is more than the others.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice A. This data indicates that force increases non-linearly with displacement.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Refer to the following passage to answer the next five questions.
Consider an example of continuous data. Jamie is doing an experiment in physics class. In the experiment, he is trying to answer the question, “How does the velocity of a ball rolling down a ramp change with time?”
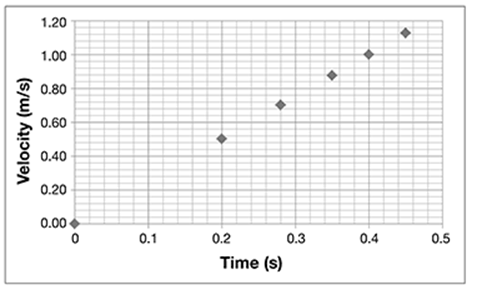
Distances along the ramp are marked at 0.1, 0.2, 0.3, 0.4, and 0.5 m. He rolls a ball down the ramp. He starts a stopwatch when he releases the ball at the top of the ramp and stops it when it passes the first mark. He repeats this four times and records the average time. He repeats the experiment again, but this time, he stops the watch at the second mark and records the time. He continues with the procedure until he reaches the last mark. He then records the average times and calculates the velocities. The data are shown in the table.
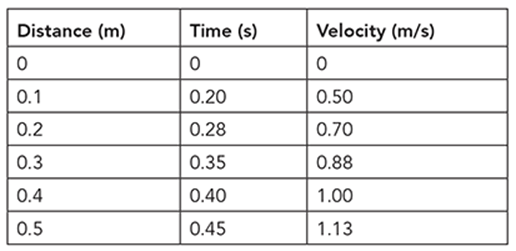
Using the graph, plot points for distance following the data given in the table.
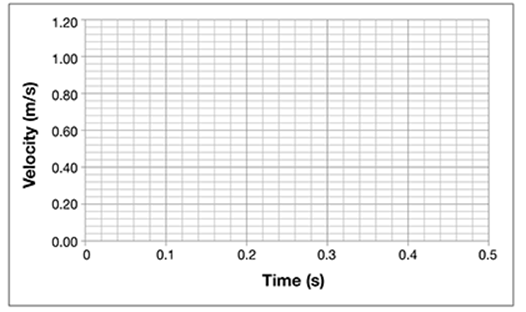
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What pattern do the data points make on the graph?

. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What can Jamie conclude about how the velocity of the rolling ball changes?

. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The data points seem to form a line. Draw a line that fits through the data points. It should look like this.
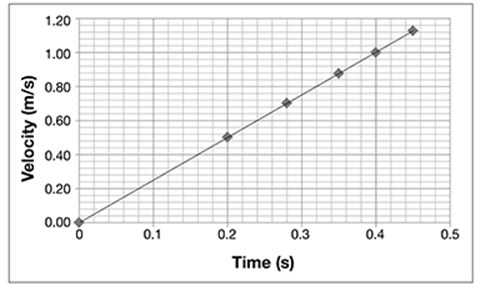
This is called a line graph.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
As time increases, so does the velocity of the ball. Furthermore, the increase forms a straight line (linear), which means it occurs at a constant rate.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Which is the independent variable?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Which is the dependent variable?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Time is the independent variable. Ask yourself—does the velocity of the ball change time? No. Therefore, time must be the independent variable.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
If the velocity of the ball does not change time, then time may change the velocity of the ball. Therefore, the velocity of the ball is the dependent variable.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Cindy is conducting an experiment on human hearing and musical notes. She blindfolds a subject and plays a reference note like middle C (C4). Next, she plays another note and asks the subject whether the note was higher or lower relative to the reference note. She indicates a higher pitch with one or more plus signs and a lower note with one or more minus signs. She organizes her subject’s responses in a table like this:
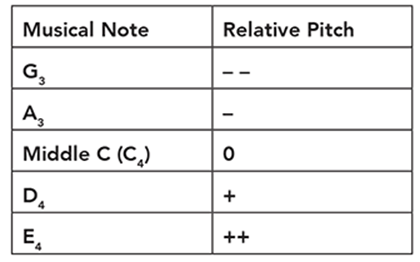
Using the following data, what is the proper order from lowest note to the highest note?
• A3
• C4
• D4
• E4
• G3
Lowest ____, ____, ____, ____, ____ Highest
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Lowest G3, A3, C4, D4, E4 Highest
We can also represent the order with mathematical signs such as G3 < A3 < C4 < D4 < E4.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Cindy is conducting an experiment on human hearing and musical notes. She blindfolds a subject and plays a reference note like middle C (C4). Next, she plays another note and asks the subject whether the note was higher or lower relative to the reference note. She indicates a higher pitch with one or more plus signs and a lower note with one or more minus signs. She organizes her subject’s responses in a table like this:
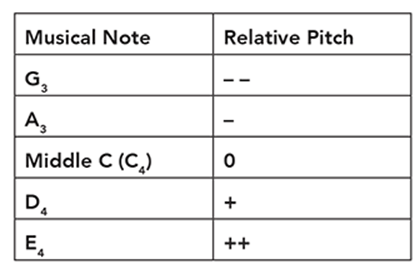
Do we know how much higher musical note E4 is than musical note G3, and how do we know this?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Jill is watching the development of red color in a solution with time during a chemical reaction. She notes the time and rates the color on a scale of 1 to 10 with 1 being light red (almost pink) and 10 being dark red (almost purple). She expresses her data in a table.
After 1 minute, what color is the solution?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Answers may vary.
No, all we know is the relative pitch. This is a drawback to qualitative data.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The solution is light red, (almost pink).
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Jill is watching the development of red color in a solution with time during a chemical reaction. She notes the time and rates the color on a scale of 1 to 10 with 1 being light red (almost pink) and 10 being dark red (almost purple). She expresses her data in a table.
Which color does the solution finally develop into?
• almost pink
• red
• almost purple
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
red
The solution develops a red, perhaps slightly dark red color, as indicated by the middle number of the scale (5 or 6).
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A biochemist conducts an experiment in which she measures the rate of a reaction as a function of temperature. The reaction is conducted in the presence and absence of an enzyme. She also monitors the pH of the reaction. Here are the data:
Which data are more important?
• rate versus temperature
• pH versus temperature
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
rate versus temperature
The biochemist would want to show the rate versus temperature data, as that is what is changing.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A biochemist conducts an experiment in which she measures the rate of a reaction as a function of temperature. The reaction is conducted in the presence and absence of an enzyme. She also monitors the pH of the reaction. Here are the data:


How would you simplify this presentation?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Answers may vary.
The pH of the reaction does not significantly change with temperature, and could be easily stated verbally as the pH of the reaction mixture was constant at approximately 7.20.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The graph describes the change in motion of a car.
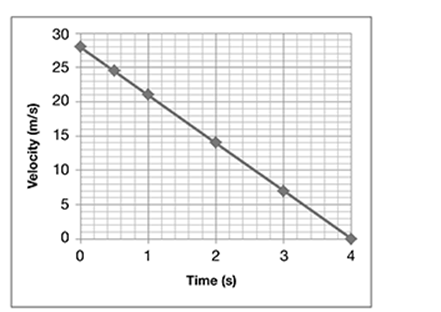
Which statement describes the results?
A. The velocity increases linearly at a rate of seven m/s/s.
B. The velocity increases non-linearly at a rate of seven m/s/s.
C. The velocity decreases non-linearly at a rate of seven m/s/s.
D. The velocity decreases linearly at a rate of seven m/s/s.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice D. The velocity is decreasing linearly at a constant rate of seven m/s/s.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A student observes a chemical reaction in a series of tubes. After five minutes of reaction time, she rates the development of blue color on a scale of 1 to 5 (1 = pale blue, 5 = navy blue). The results are shown in the table.
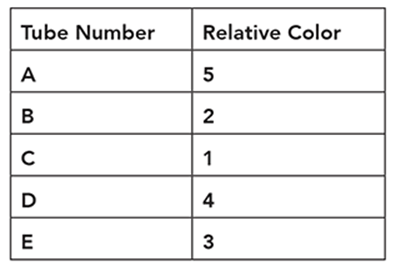
Which represents the series of tubes in order from least color development to most color development?
A. A < B < C < D < E
B. C < B < E < D < A
C. E < D < A < B < C
D. E < D < C < B < A
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice B. The tubes are in the correct order from least color development (Tube C = 1) to most color development
(Tube A = 5).
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
In a physics lab, a student heats an iron block. He measures the temperature change and mass of the block as it heats up. He calculates the thermal energy transferred to the block. The results are shown in the following graphs.
Which best describes the experimental results in words?
A. The thermal energy decreases at a constant rate, while the mass stays constant.
B. The thermal energy increases at a constant rate, while the mass stays constant.
C. The thermal energy decreases at a constant rate, while the mass increases.
D. The thermal energy increases at a constant rate, while the mass decreases.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice B. Thermal energy increases linearly (constant rate), while mass remains constant.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Jenny does an experiment with her friend to test her friend’s ability to hear loud (indicated by positives) or soft (indicated by negatives) sounds relative to a reference sound. The data are shown in the table.
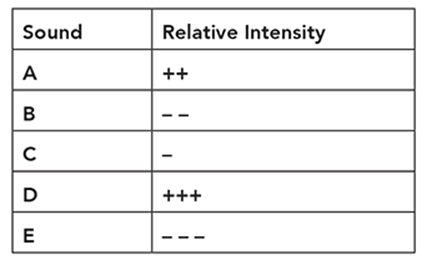
Which sound was closest to the reference sound?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Which graph can be described as decreasing force (F) as a non-linear function of displacement (x)?

A. graph A
B. graph B
C. graph C
D. graph D
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Sound C had only one negative and was the closest to the reference sound.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Graph D. This data indicates that force decreases non-linearly with displacement.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A man is 2.0 m away from you. He walks in a straight line away from you at a constant velocity. His position with time is shown in the table and the graph. What is the man’s velocity?
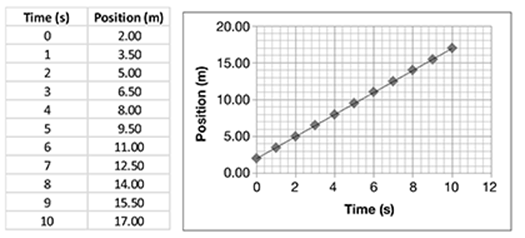
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A man is 2.0 m away from you. He walks in a straight line away from you at a constant velocity. His position with time is shown in the table and the graph.

How far away will the man be at 30 s if he keeps walking at the same velocity?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
By definition, velocity is the rate of change of position with time or the slope of the straight line on a position–time graph. It’s a straight line because he is walking at constant velocity. So, to find the equation of the line, we must first calculate the slope of the line:
1. Pick any two points on the line. We’ll use (2,5) and (4,8).
2. Use the slope equation:
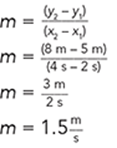
So, the man’s velocity is 1.5 m/s.
3. Now, let’s find the y-intercept. At time zero, the man started from 2 m away. Therefore, the y-intercept is 2 m. You can see this on the graph as well. It’s the point where the line crosses the y-axis (vertical axis).
4. So, the equation of the line in the form y = mx + b becomes: y = 1.5x + 2.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
47 m. Determine the velocity of the man using the slope equation using two points like (2,5) and (4,8).
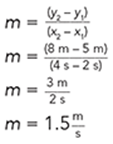
So, the man’s velocity is 1.5 m/s.
The time is 30 s, so x = 30. Use the equation:
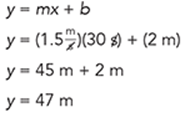
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A student is following a chemical assay to measure the amount of protein in a sample. The reaction turns blue in the presence of protein. She runs known solutions of protein plus an unknown and measures the amount of blue color or absorbance in a device called a spectrophotometer. The results are shown in the table and graph.
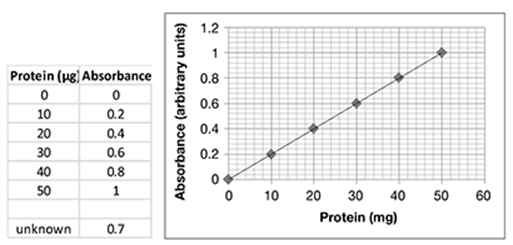
The line formed by the data from the protein standards is called a standard curve.
What is the equation of the standard curve?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
y = 0.02x. The equation of the standard curve is y = 0.02x. Here’s the solution. Use two points on the line, like (30,0.6) and (10,0.2), and find the slope:
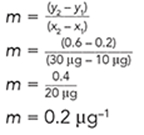
The y-intercept is zero, so the equation for the standard curve is y = 0.02x.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A student is following a chemical assay to measure the amount of protein in a sample. The reaction turns blue in the presence of protein. She runs known solutions of protein plus an unknown and measures the amount of blue color or absorbance in a device called a spectrophotometer. The results are shown in the table and graph.

The line formed by the data from the protein standards is called a standard curve.
How much protein is in the unknown sample?
____ µg
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
35 µg. There is 35 µg of protein in the unknown sample. You have the y-value of 0.7, so solve the equation for x:
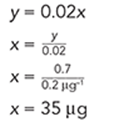
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A sound wave has a frequency of 262 Hz. It travels at 340 m/s. What is the wavelength of the sound wave? (If you do not remember the formula, it is v = fλ where v is velocity, f is frequency, and λ is the wavelength.)
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
1.30 m. The answer is 1.30 m. Here’s the solution (remember that the unit Hz is cycles per second or s–1):
Given: f = 262 Hz, ν = 340 m/s
Unknown: λ = ?

Review the answer. Does it make sense?
Yes, the answer makes sense. The unit is correct and sound waves are rather large (i.e., on the order of meters long).
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Refer to the following passage to answer the next four questions.
A worker on an assembly line in a chocolate factory samples 10 candy bars every hour to make sure that the candy bars produced meet specifications. The specifications of the candy bars state that the candy bar length must be 15 cm ± 0.5 cm. The worker obtains this data set:
15.1, 14.9, 15.0, 14.6, 15.0, 15.0, 15.0, 14.9, 15.4, 14.9
What is the mean of the sample data set?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is the median of the sample data set?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is the mode of the sample data set?
14.98 cm. The mean is 14.98 cm, the median is 15.0 cm, and the mode is 15.0 cm. First let’s arrange the numbers in ascending order:
14.6, 14.9, 14.9, 14.9, 15.0, 15.0, 15.0, 15.0, 15.1, 15.4
The mean is calculated like this:
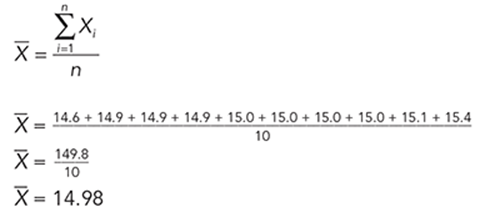
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
15.0 cm.
First let’s arrange the numbers in ascending order:
14.6, 14.9, 14.9, 14.9, 15.0, 15.0, 15.0, 15.0, 15.1, 15.4
There is an even number, so the median is the average of the two middle values. The average of 15 and 15 is 15. So, the median is 15.0 cm.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
15.0 cm.
First let’s arrange the numbers in ascending order:
14.6, 14.9, 14.9, 14.9, 15.0, 15.0, 15.0, 15.0, 15.1, 15.4
To calculate the mode, list each value and its frequency in a table.
You see that 15.0 cm has the highest frequency, so the mode is 15.0 cm.
Do the candy bars meet length specifications for that hour?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The graph describes the change in motion of a car.

Which equation models the car’s motion?
A. y = 7x + 28
B. y = 28x + 7
C. y = –28x + 7
D. y = –7x + 28
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Yes. All descriptors (mean, median, and mode) are in the range of 15.0 cm ± 0.5 cm, as are all the individual candy bars (14.6 cm to 15.4 cm). Therefore, the candy bars meet the specifications for that hour.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice D. The graph shows that the velocity is decreasing linearly and can be described by the equation y = –7x + 28.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A man pushes a box horizontally across a frictionless surface for 15 m. He does 150 J of work. How much net force did he apply to the box?
A. 15 N
B. 10 N
C. 150 N
D. 1.0 N
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
In a physics experiment, a student makes several measurements of the velocity of a sound wave from a tuning fork. The measurements (in m/s) are:
341, 343, 330, 335, 338, 345, 341
What is the mean of the sample data set?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A molecular biologist wanted to make an artificial peptide containing three amino acids (there are 20 possible naturally occurring amino acids that he could use). Without repeating any amino acids, how many permutations are there?
A. 1,140
B. 6,840
C. 7,980
D. 3.2 million
The correct answer is choice B. Here’s the solution:
Given: W = 150 J, d = 15 m
Unknown: Fnet = ?

. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
339. Here’s the solution:
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice B. Here’s the solution:
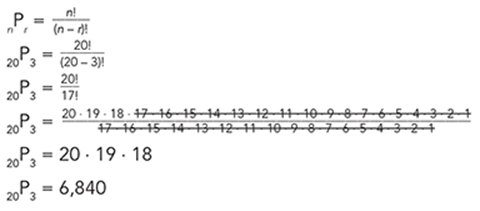
A student observes a chemical reaction in a series of tubes. After 5 minutes of reaction time, she rates the development of blue color on a scale of 1 to 5 (1 = pale blue, 5 = navy blue). The results are shown in the table.
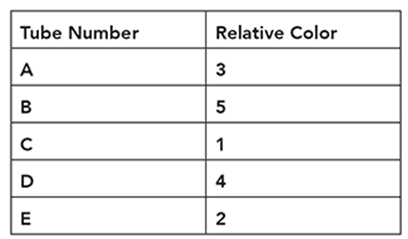
Which represents the series of tubes in order from least color development to most color development?
A. A < B < C < D < E
B. C < E < A < D < B
C. E < D < A < B < C
D. E < D < C < B < A
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice B. The tubes are in the correct order from least color development (Tube C = 1) to most color development
(Tube B = 5).
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A chemistry student conducts an investigation on the behavior of gases. He knows that pressure, volume, and temperature are variables and keeps the amount of gas constant. In three experiments, he keeps one variable fixed, while he varies another and measures the third. The results are plotted on graphs.
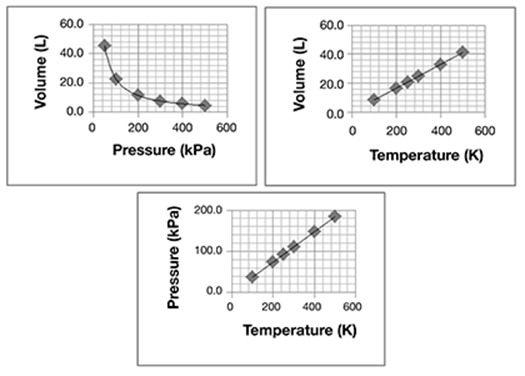
Which conclusions about gas behavior are true?
A. Temperature is inversely related to pressure and directly related to volume, while pressure is inversely related to volume.
B. Pressure is inversely related to both volume and temperature, while volume is directly related to temperature.
C. Volume is inversely related to pressure and directly related to temperature, while pressure is directly related to temperature.
D. The amount of gas is directly related to pressure and temperature, but inversely related to volume.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice C. The first graph shows that volume is inversely related to pressure, the second graph shows that volume is directly related to temperature, and the third graph shows that pressure is directly related to temperature.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Which set of data accurately describes a car’s motion under constant deceleration?
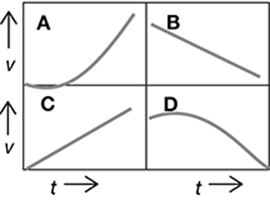
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A clinical researcher has learned that five slots have opened up in a clinical drug trial. Fifty applicants are eligible for the positions. Which is the possible number of permutations?
A. 250
B. 2,118,760
C. 254,251,200
D. 15,625,000,000
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A 2,000-kg truck accelerates at 40,000 m/s2. What is the force acting on the truck?
A. 200 N
B. 40,000 N
C. 40 million N
D. 80 million N
The correct answer is set B. This data indicates the car is decelerating at a constant rate, the slope of the line.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice C. Here’s the solution.

. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice D. Here’s the solution:
Given: a = 40,000 m/s2, m = 2,000 kg
Unknown: Fnet = ?

A chemistry student conducts an experiment in which he keeps the temperature and volume of a gas constant. He increases the amount of the gas and measures the pressure. The data are shown in the table. He creates a graph in his lab report and the teacher marks it wrong.
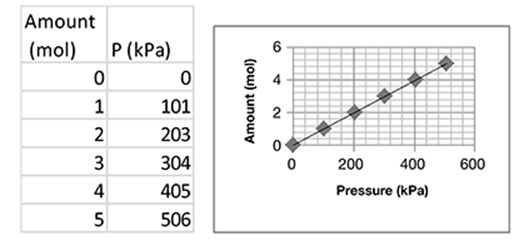
Which error did the student commit in making the graph?
A. The x-axis is not graded in even increments.
B. The y-axis is not graded in even increments.
C. There should be no line through the data points.
D. The variables are plotted on the wrong axes.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice D. Amount is the independent variable and should be plotted on the x-axis. In contrast, pressure is the dependent variable and should be plotted on the y–axis.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Which of the graphs correctly depicts constant positive velocity?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Work is a force acting on an object to move it across a distance. Inclined planes make work easier by providing a smooth surface for objects to slide or roll across. Pushing a stroller up a ramp is easier than pushing it up a flight of stairs. When going down a ramp, the gravitational pull on the stroller may be all that the stroller needs to begin rolling and gain velocity.
The velocity of a moving object can be determined by the following formula:
![]()
If a stroller travels with a forward velocity of four m/s for a time of two seconds, then the distance covered is ___________ meters. (You may use a calculator to complete this question.)
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Graph A is the correct one. Velocity is speed with direction, and it is calculated by dividing the distance traveled by the time it took to cover that distance. On this graph, time is increasing to the right, and position is increasing constantly with time. At any point on graph A, the position divided by time will produce the same number, indicating constant positive velocity.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is eight meters. The formula for velocity, ν = ![]() , can be rearranged to solve for distance. In this case, d = ν × t, which is four m/s multiplied by two seconds.
, can be rearranged to solve for distance. In this case, d = ν × t, which is four m/s multiplied by two seconds.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Which of the following materials would be a good insulator?
A. a tile floor because it transfers heat away from skin
B. a steel spoon because it conducts heat from boiling liquids
C. a wool blanket because it slows the transfer of heat from skin
D. a copper pipe because it accelerates the transfer of heated materials
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Every chemical reaction needs a certain amount of energy to get started, as illustrated in the graph.
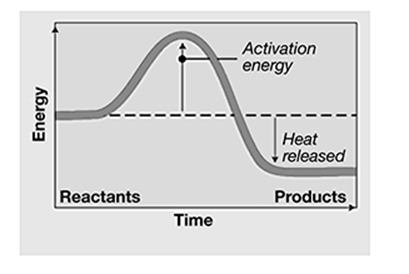
What type of reaction is shown?
A. endothermic, because energy is required after activation to continue the reaction
B. exothermic, because additional energy is needed in order to complete the reaction
C. exothermic, because the energy level of the products is lower than the energy level of the starting materials
D. endothermic, because the energy level of the final materials is higher than that of the starting materials
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice C. Materials such as wool are good insulators because they are poor conductors of heat. A wool blanket will slow the transfer of heat from the body so that it feels warmer.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice C. The graph shows that additional energy is not needed to complete the reaction; energy is given off as the reaction takes place. As a result, the energy level of the products is lower than the energy level of the starting materials.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The amount of kinetic energy a moving object has depends on its velocity and mass. Kinetic energy can be calculated by using the following formula:
![]()
Which of the following would have the most kinetic energy?
A. a truck driving 10 m/s
B. a bicycle traveling 10 m/s
C. a car stopped at a red light
D. a school bus parked on a hill
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Almost all the weight of a carbon atom comes from which of these particles?
A. protons only
B. neutrons and electrons
C. protons and neutrons
D. protons and electrons
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice A. While a truck and a school bus may have similar mass, the school bus is parked, indicating a velocity of zero. Thus, the truck moving at any velocity will have the highest kinetic energy. Similarly, even though the bicycle is in motion, the greater mass of the truck will contribute to its larger kinetic energy.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice C. Almost all the weight of an atom comes from the protons and neutrons in its nucleus. Neutrons weigh exactly one atomic mass unit, and protons weigh almost one atomic mass unit (1.67 × 10–24grams).
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Examine the diagram of an atom shown here.
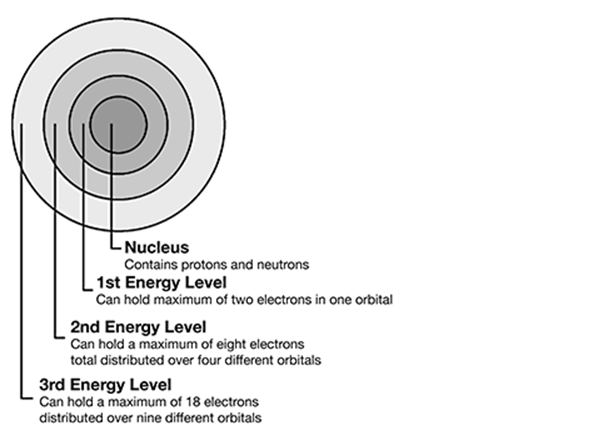
Which of these would be found in the first energy level?
A. eight neutrons
B. 18 electrons
C. protons and neutrons
D. no more than two electrons
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice D. The first energy level of an atom can hold a maximum of two electrons in one orbital. Each energy level is capable of holding a specific number of electrons.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A cleaning service wants to offer a natural alternative to the industrial products it normally uses. Instead of bleach, the company uses a mixture of vinegar and baking soda. When the liquid vinegar and powdered baking soda combine, a bubbly gas is produced. What chemical property is observed?
A. flammability
B. color change
C. volume
D. reactivity
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice D, reactivity. Reactivity is the tendency of a substance to undergo a chemical reaction, either by itself or with other materials, and to release energy. Reactivity with other chemicals is evidenced when the baking soda and vinegar combine and react to create carbon dioxide gas, as seen by the bubbles.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The following chart lists the mechanical properties of different metals and alloys.
A jewelry designer wants to work with new types of materials. She needs a metal that is easy to shape, is not easily broken, and is resistant to tarnishing. Based on the chart, which material would be the best choice?
A. gold
B. nickel
C. bismuth
D. manganese
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice A, gold, because it tops the list for corrosion resistance and will not tarnish. It is also malleable, so it is easy to shape, but will not break easily because it is not brittle.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Hydrogen peroxide (H2O2) is stored in dark, opaque containers to slow the natural breakdown of the compound.
The reaction is summarized by this formula:
2 H2O2 → _____ H2O + O2
Write the missing number of water molecules on the line.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
In a chemical formula, subscripts show the ratio of one kind of atom to another. For example, NH3 shows that there are three hydrogen atoms for every one nitrogen atom.
Examine the following ratios:
• twice as many sodium atoms as carbon atoms
• three times as many oxygen atoms as carbon atoms
Which chemical formula correctly shows the ratios described?
A. Na2CO3
B. NaCO3
C. Na3CO2
D. Na6CO12
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is velocity?
2 H2O2 → 2 H2O + O2
The breakdown of hydrogen peroxide into water and oxygen is summarized as 2 H2O2 → 2 H2O + O2. The two balances the equation because there are two water molecules to equal four hydrogens.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The correct answer is choice A, Na2CO3. This formula shows sodium with a subscript of 2, indicating twice as many atoms as the one carbon atom. Oxygen has a subscript of 3, three times more than the single carbon atom.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The rate that a position changes in a constant direction per unit of time. Common units are meters per second (m/s).
What is the definition of volume?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is valence?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is a transverse wave?
A cubic measurement that measures how many cubic units it takes to fill a solid figure:
• Cube: V = s3, where s is the length of any edge of the cube.
• Rectangular solid: V = length × width × height
• Square pyramid: V = 1/3 × (base edge)2 × height
• Cone: V = 1/3 × ð × radius2 × height; ð is approximately equal to 3.14.
• Cylinder: V = ð × radius2 × height; ð is approximately equal to 3.14
• Prism: V = B × h (the area of the base × the height)
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The combining power of an element as shown by the number of electrons that are in the outer atomic shell and can participate in a chemical reaction.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A wave in which the motion of the particles in the medium is perpendicular to the direction of wave propagation. Light is an example of a transverse wave.
What is the definition of tension?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is a spontaneous reaction?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is the speed of light?
The force that acts on and is transferred along ropes, strings, and chains.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A reaction that does not require an external source of energy to proceed.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The speed of light in a vacuum (186,282 miles per second) is the fastest speed possible. As light travels in other materials, it will change speed. The speed of light in any material is still the fastest speed possible in that material. Commonly denoted by the symbol c.
What is a series circuit?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
What is a reversible reaction?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A circuit with only one path for an electrical current to follow. The current in each element in a series circuit is the same.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A reaction in which products can revert back into reactants.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Refer to the following passage to answer the next four questions.
A student is conducting an experiment involving inertia with a dozen eggs, one of which is hard-boiled. Inertia is defined as the property of matter by which it resists any change in velocity. The student spins each egg and then touches it to stop the egg from moving. The student observes that the hard-boiled egg spins and stops easily as it is touched, but the raw eggs continue moving even after being touched to stop the spin.
Why does the hard-boiled egg stop spinning so easily?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Why do the raw eggs continue trying to move?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
How does this experiment reference inertia?
The mass inside the egg is solid and evenly distributed.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The liquid portion inside the egg causes a drag effect that resists the spin initially and then resists being made motionless at the end.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Since inertia is defined by resistance to change in velocity, it can be determined that a raw egg has a greater tendency toward inertia due to its liquid portion than a hard-boiled egg.
What variable of this experiment has not been fully determined?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
The surface on which the eggs are spun. In order to determine that the surface is not contributing to inertia on either the hard-boiled egg or the raw eggs, the surface should be smooth and frictionless.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .