CONCEPTS IN BIOLOGY
PART II. CORNERSTONES: CHEMISTRY, CELLS, AND METABOLISM
5. Enzymes, Coenzymes, and Energy
5.5. Cellular-Control Processes and Enzymes
In any cell, there are thousands of kinds of enzymes. Each controls specific chemical reactions and is sensitive to changing environmental conditions, such as pH and temperature. For a cell to stay alive in an ever-changing environment, its countless chemical reactions must be controlled. Recall from chapter 1 that control processes are mechanisms that ensure that an organism will carry out all metabolic activities in the proper sequence (coordination) and at the proper rate (regulation). The coordination of enzymatic activities in a cell results when specific reactions occur in a given sequence— for example, A → B → C → D → E. This ensures that a particular nutrient will be converted to a particular end product necessary to the survival of the cell. Should a cell be unable to coordinate its reactions, essential products might be produced at the wrong time or never be produced at all, and the cell would die. The regulation of biochemical reactions is the way a cell controls the amount of chemical product produced. The expression “having too much of a good thing” applies to this situation. For example, if a cell manufactures too much lipid, the presence of those molecules could interfere with other life-sustaining reactions, resulting in the cell’s death. On the other hand, if a cell does not produce enough of an essential molecule, such as a hydrolytic (digestive) enzyme, it might also die. The cellular-control process involves both enzymes and genes.
Enzymatic Competition for Substrates
Enzymatic competition results whenever there are several kinds of enzymes available to combine with the same kind of substrate molecule. Although all these different enzymes may combine with the same substrate, they do not have the same chemical effect on the substrate, because each converts the substrate to different end products. For example, acetyl- coenzyme A (acetyl-CoA) is a substrate that can be acted upon by three different enzymes: citrate synthetase, fatty acid synthetase, and malate synthetase (figure 5.7). Which enzyme has the greatest success depends on the number of each type of enzyme available and the suitability of the environment for the enzyme’s operation. The enzyme that is present in the greatest number or is best suited to the job in the environment of the cell wins, and the amount of its end product becomes the greatest.
Gene Regulation
The number and kind of enzymes produced are regulated by the cell’s genes. It is the job of chemical messengers to inform the genes as to whether specific enzyme-producing genes should be turned on or off, or whether they should have their protein-producing activities increased or decreased. Gene-regulator proteins are chemical messengers that inform the genes of the cell’s need for enzymes. Gene-regulator proteins that decrease protein production are called gene-repressor proteins, whereas those that increase protein production are gene-activator proteins. Look again at figure 5.7. If the cell were in need of protein, gene-regulator proteins could increase the amount of malate synthetase. This would result in an increase in the amount of acetyl-CoA being converted to malate. The additional malate would then be modified into one of the amino acids needed to produce the needed protein. On the other hand, if the cell required energy, an increase in the amount of citrate synthetase would cause more acetyl-CoA to be metabolized to release this energy. When the enzyme fatty acid synthetase is produced in greater amounts, it outcompetes the other two; the acetyl-CoA is used in fat production and storage.
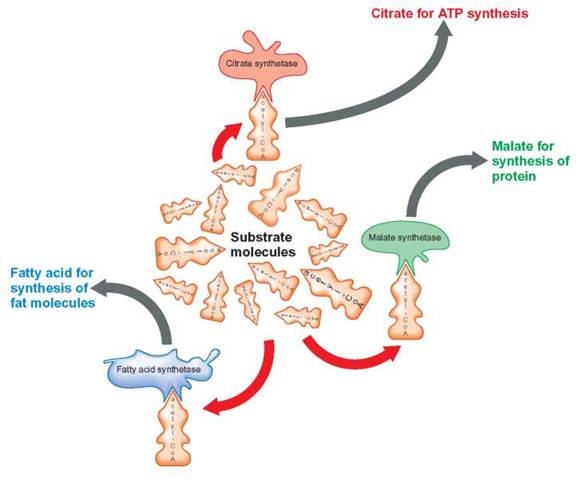
FIGURE 5.7. Enzymatic Competition
Acetyl-CoA can serve as a substrate for a number of reactions. Three such reactions are shown here. Whether it becomes a fatty acid, malate, or citrate is determined by the enzymes present. Each of the three enzymes can be thought of as being in competition for the same substrate—the acetyl-CoA molecule. The cell can partially control which end product will be produced in the greatest quantity by producing greater numbers of one kind of enzyme and fewer of the other kinds. If citrate synthetase is present in the highest quantity, more of the acetyl-CoA substrate will be acted upon by that enzyme and converted to citrate, rather than to the other two end products, malate and fatty acids.
Inhibition
An inhibitor is a molecule that attaches itself to an enzyme and interferes with that enzyme’s ability to form an enzyme- substrate complex (How Science Works 5.1). For example, one of the early kinds of pesticides used to spray fruit trees contained arsenic. The arsenic attached itself to insect enzymes and inhibited the normal growth and reproduction of insects. Organophosphates are pesticides that, at the right concentration, inhibit several enzymes necessary for the operation of the nervous system. When they are incorporated into nerve cells, they disrupt normal nerve transmission and cause the death of the affected organisms (figure 5.8). In humans, death that is due to pesticides is usually caused by uncontrolled muscle contractions, resulting in breathing failure.
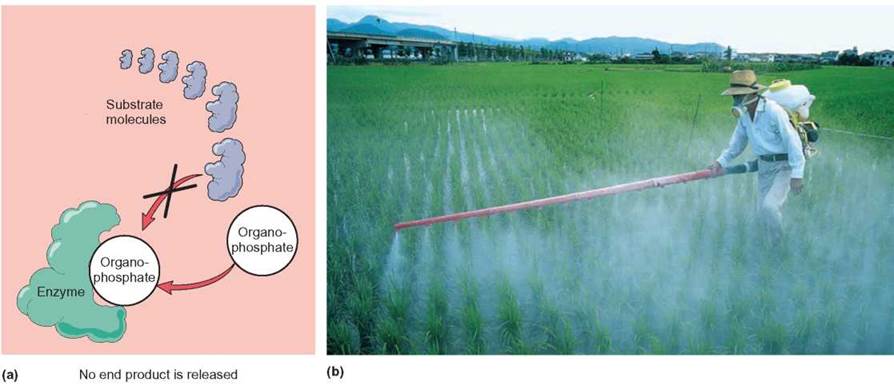
FIGURE 5.8. Inhibition of Enzyme at Active Site
(a) Organophosphate pesticides are capable of attaching to the enzyme acetylcholinesterase, preventing it from forming an enzyme-substrate complex with its regular substrate. Since acetylcholinesterase is necessary for normal nerve cell function, organophosphates pesticides are nerve poison and kills organisms. (b) Many farmers around the world use organophosphates to control crop-damaging insects.
Competitive Inhibition
Some inhibitors have a shape that closely resembles the normal substrate of the enzyme. The enzyme is unable to distinguish the inhibitor from the normal substrate, so it combines with either or both. As long as the inhibitor is combined with an enzyme, the enzyme is ineffective in its normal role. Some of these enzyme-inhibitor complexes are permanent. An inhibitor removes a specific enzyme as a functioning part of the cell. The reaction that enzyme catalyzes no longer occurs, and none of the product is formed. This is termed competitive inhibition because the inhibitor molecule competes with the normal substrate for the active site of the enzyme (figure 5.9).
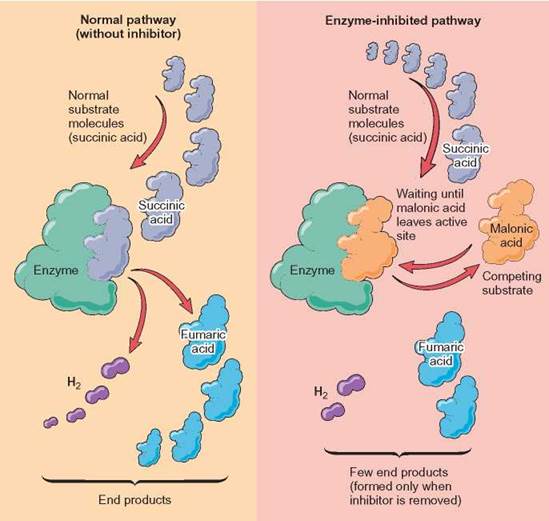
FIGURE 5.9 Competitive Inhibition
The left-hand side of the illustration shows the normal functioning of the enzyme. On the right- hand side, the enzyme is unable to attach to succinic acid. This is because an inhibitor, malonic acid, is attached to the enzyme and prevents the enzyme from forming the normal complex with succinic acid. As long as malonic acid stays attached in the active site, the enzyme will be unable to produce fumaric acid. If the malonic acid is removed, the enzyme will begin to produce fumaric acid again. Its attachment to the enzyme in this case is not permanent but, rather, reduces the number of product molecules formed per unit of time, its turnover number.
Scientists use their understanding of enzyme inhibition to control disease. For instance, an anti-herpes drug is used to control herpes viruses responsible for lesions such as genital herpes or cold sores. The drug Valtrex inhibits the viral form of the enzyme DNA polymerase that is responsible for the production of compounds required for viral replication. As a result, the viruses are unable to replicate and cause harm to their host cells. Because people do not normally produce this enzyme, they are not harmed by this drug.
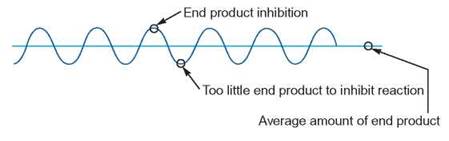
Negative-Feedback Inhibition
Negative-feedback inhibition is another method of controlling the synthesis of many molecules within a cell. This control process occurs within an enzyme-controlled reaction sequence. As the number of end products increases, some product molecules feed back to one of the previous reactions and have a negative effect on the enzyme controlling that reaction; that is, they inhibit, or prevent, that enzyme from performing at its best.
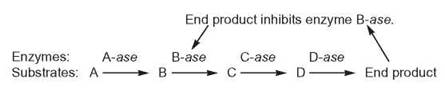
If the enzyme is inhibited, the end product can no longer be produced at the same rapid rate, and its concentration falls. When there are too few end product molecules to have a negative effect on the enzyme, the enzyme is no longer inhibited. The enzyme resumes its previous optimum rate of operation, and the end product concentration begins to increase. With this kind of regulation, the amount of the product rises and falls within a certain range and never becomes too large or small.
HOW SCIENCE WORKS 5.1
Don't Be Inhibited—Keep Your Memory Alive
Alcohol and drugs can interfere with your "short-term" memory, such as remembering the crazy things you might have done at a party Saturday night. However, they don't seem to get in the way of older memories, such as the biology exam you failed in high school. Neuroscientists thought this is because long-term memories become "hard-wired" into your brain in a way that makes them harder to wipe out. These long-term memories are kept in place by structural changes to the connections between nerve cells, but recent research has made this "simple" explanation more complicated.
The research involved injecting a drug that inhibits the enzyme protein kinase into the cerebral cortex of rat brains where taste memories are thought to reside.
The data revealed that when this enzyme was blocked, the rats forgot a meal that made them sick weeks earlier. The results of these experiments suggest that the continuous activity of this enzyme is somehow necessary to maintain long-term memory. This is something that was not predicted by the hypotheses on the mechanisms of memory formation. Protein kinase and other similar enzymes were thought to only be important in the early stages of memory formation. Now it appears that they are needed to form and sustain long-term memory. One researcher at the University of Arizona in Tucson believes that it's possible that protein kinase can erase all learning, no matter how long it has been stored in memory.
What does the future have in store for the therapeutic applications of such research? Some are thinking about the development of enzyme-altering drugs that could:
• help sustain memories for longer than normal periods,
• boost brainpower, and
• eliminate the painful memories of trauma survivors.
5.5. CONCEPT REVIEW
13. What is enzyme competition, and why is it important to all cells?
14. Describe the nature and action of an enzyme inhibitor.