CONCEPTS IN BIOLOGY
PART II. CORNERSTONES: CHEMISTRY, CELLS, AND METABOLISM
2. The Basics of Life
2.5. Physical Changes—Phases of Matter
There are implications to the kinetic molecular theory. First, the amount of kinetic energy that particles contain can change. Molecules can gain from or lose energy to their surroundings, resulting in changes in their behavior. Second, molecules have an attraction for one another. This force of attraction is important in determining the phase in which a particular kind of matter exists.
The amount of kinetic energy molecules have, the strength of the attractive forces between molecules, and the kind of arrangements they form result in three phases of matter: solid, liquid, and gas (figure 2.7). A solid (e.g., bone) consists of molecules with strong attractive forces and low kinetic energy. The molecules are packed tightly together. With the least amount of kinetic energy of all the phases of matter, these molecules vibrate in place and are at fixed distances from one another. Powerful forces bind them together. Solids have definite shapes and volumes under ordinary temperature and pressure conditions. The hardness of a solid is its resistance to forces that tend to push its molecules farther apart. There is less kinetic energy in a solid than in a liquid of the same material.
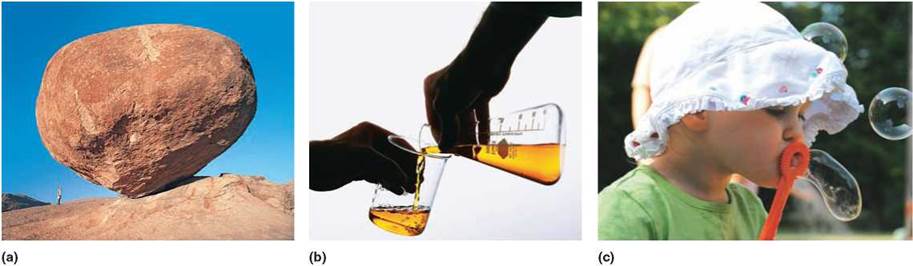
FIGURE 2.7. Phases of Matter
(a) In a solid, such as this rock, molecules vibrate around a fixed position and are held in place by strong molecular forces. (b) In a liquid, molecules can rotate and roll over each other, because the kinetic energy of the molecules is able to overcome the molecular forces. (c) Inside the bubble, gas molecules move rapidly in random, free paths.
A liquid (e.g., the water component of blood and lymph) has molecules with enough kinetic energy to overcome the attractive forces that hold molecules together. Thus, although the molecules are still strongly attracted to each other, they are slightly farther apart than in a solid. Because they are moving more rapidly, and the attractive forces can be overcome, they sometimes slide past each other. Although liquids can change their shape under ordinary conditions, they maintain a fixed volume under ordinary temperature and pressure conditions—that is, a liquid of a certain volume will take the shape of the container into which it is poured, but it will take up the same amount of space regardless of the container’s shape. This gives liquids the ability to flow, so they are called fluids.
A gas (e.g., air) is made of molecules that have a great deal of kinetic energy. The attraction the gas molecules have for each other is overcome by the speed with which the individual molecules move. Because gas molecules are moving faster than the molecules of solids or liquids, their collisions tend to push them farther apart, so a gas expands to fill its container. The shape of the container and the pressure determine the shape and volume of the gas. The term vapor is used to describe the gaseous form of a substance, that is normally in the liquid phase. For example, water vapor is the gaseous form of liquid water and mercury vapor in CFLs is the gaseous form of liquid mercury (How Science Works 2.2).
2.5. CONCEPT REVIEW
12. Which phase of matter is composed of molecules that vibrate around a fixed position and are held in place by strong molecular forces?
13. Which phase of matter is composed of molecules that can rotate and roll over each other because the kinetic energy of the molecules is able to overcome the molecular forces?
14. Which phase of matter is composed of atoms or molecules with the greatest amount of kinetic energy?