THE LIVING WORLD
Unit two. The Living Cell
2. The Chemistry of Life
2.2. Ions and Isotopes
Ions
Sometimes an atom may gain or lose an electron from its outer shell. Atoms in which the number of electrons does not equal the number of protons because they have gained or lost one or more electrons are called ions. All ions are electrically charged. For example, an atom of sodium (on the left in figure 2.5) becomes a positively charged ion, called a cation (on the right), when it loses an electron, such that one proton in the nucleus is left with an unbalanced charge (11 positively charged protons and only 10 negatively charged electrons). Negatively charged ions, called anions, also form when an atom gains an electron from another atom.
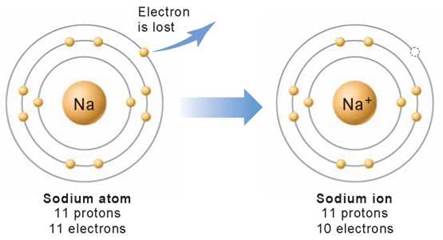
Figure 2.5. Making a sodium ion.
An electrically neutral sodium atom has 11 protons and 11 electrons. Sodium ions bear one positive charge when they ionize and lose one electron. Sodium ions have 11 protons and only 10 electrons.
Isotopes
The number of neutrons in an atom of a particular element can vary without changing the chemical properties of the element. Atoms that have the same number of protons but different numbers of neutrons are called isotopes. Isotopes of an atom have the same atomic number but differ in their mass number. Most elements in nature exist as mixtures of different isotopes. For example, there are three isotopes of the element carbon, all of which possess six protons (the purple balls in figure 2.6). The most common isotope of carbon (99% of all carbon) has six neutrons (the pink balls). Because its mass number is 12 (six protons plus six neutrons), it is referred to as carbon-12. The isotope carbon-14 (on the right) is rare (1 in 1 trillion atoms of carbon) and unstable, such that its nucleus tends to break up into particles with lower atomic numbers, a process called radioactive decay. Radioactive isotopes are used in medicine and in dating fossils.
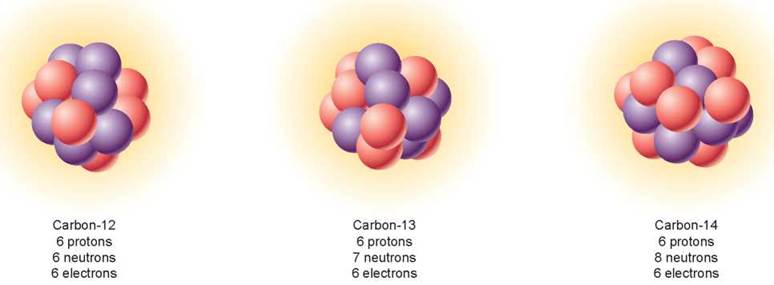
Figure 2.6. Isotopes of the element carbon.
The three most abundant isotopes of carbon are carbon-12, carbon-13, and carbon-14. The yellow "clouds" in the diagrams represent the orbiting electrons, whose numbers are the same for all three isotopes. Protons are shown in purple, and neutrons are shown in pink.
Medical Uses of Radioactive Isotopes
When most people hear the word “radioactive” they picture atomic bombs exploding into mushroom clouds and the devastation that results. While it is true that the radiation emitted from radioactive isotopes can damage cells of the human body, it is also true that isotopes can be used in many medical procedures. Short-lived isotopes, those that decay fairly rapidly and produce harmless products, are commonly used as tracers in the body. A tracer is a radioactive substance that is taken up and used by the body. Emissions from the radioactive isotope tracer are detected using special laboratory equipment, and can reveal key diagnostic information about the functioning of the body. For example, PET and PET/CT (positron emission tomography/computerized tomography) imaging procedures can be used to identify a cancerous area in the body. First, a radioactive tracer is i njected into the body. This tracer is taken up by all cells, but it is taken up in larger amounts in cells with higher metabolic activities, such as cancer cells. Images are then taken of the body, and areas emitting greater amounts of the tracer can be seen. For example, in the images in figure 2.7, the radioactive-emitting cancer site appears as a black area on the left and a yellow glowing area on the right. There are many other uses of radioactive isotopes in medicine, both in detection and treatment of disorders.

Figure 2.7. Using a radioactive tracer to identify cancer.
In certain medical imaging procedures, the patient ingests or is injected intravenously with a radioactive tracer that is absorbed in greater amounts by cancer cells. The tracer emits radioactivity that is detected using PET and PET/CT equipment. A cancerous area in the neck is seen in these two images, as a dark black area on the left and a bright yellow glowing area on the right.
Dating Fossils
Fossils are created when the remains, footprints, or other traces of organisms become buried in sand or sediment. Over time, the calcium in bone and other hard tissues becomes mineralized as the sediment is converted to rock. A fossil is any record of prehistoric life—generally taken to mean older than 10,000 years. By dating the rocks in which fossils occur, biologists can get a very good idea of how old the fossils are. Rocks are usually dated by measuring the degree of radioactive decay of certain radioactive isotopes among rock-forming minerals. A radioactive isotope is one whose nucleus is unstable and eventually flies apart, creating more stable atoms of another element. Because the rate of decay of a radioactive element (the percent of isotopes that undergo decay in a minute) is constant, scientists can use the amount of radioactive decay to date fossils. The older the fossil, the greater the fraction of its radioactive isotopes that have decayed.
A widely employed method of dating fossils less than 50,000 years old is the carbon-14 (14C) radioisotopic dating method illustrated in figure 2.8. Most carbon atoms have a mass number of 12 (12C). However, a tiny but fixed proportion of the carbon atoms in the atmosphere consists of carbon atoms with a mass number of 14 (14C). This isotope of carbon is created by the bombardment of nitrogen-14 atoms with cosmic rays. This proportion of 14C (designated A in the figure) is captured by plants in photosynthesis, and is the proportion present in the carbon molecules of the animal’s body that eats the plants, in this case a rabbit. After the plant or animal dies, it no longer accumulates carbon, and the 14C present at the time of death gradually decays over time back to nitrogen-14 (14N). The amount of 14C (A) decreases while the amount of 12C stays the same. Scientists can determine how long ago an organism died by measuring the ratio of 14C to 12C in its remains or in the fossilized rock. Over time, the ratio of 14C to 12C decreases. It takes 5,730 years for half of the 14C (1/2A or A/2) present in the sample to be converted to 14N by this process. This length of time is called the half-life of the isotope. Because the half-life of an isotope is a constant that never changes, the extent of radioactive decay allows you to date a sample. Thus a sample that had a quarter of its original proportion of 14C remaining (1/4A or A/4) would be approximately 11,460 years old (two half-lives—5,730 years for half of the 14C to decay to a level of A/2 and another 5,730 years for the remaining14C to decay to a level of A/4).
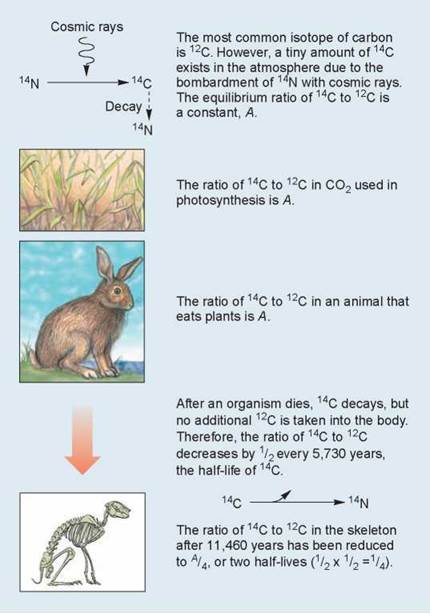
Figure 2.8. Radioactive isotope dating.
This diagram illustrates radioactive dating using carbon-14, a shortlived isotope.
For fossils older than 50,000 years, there is too little 14C remaining to measure precisely, and scientists instead examine the decay of potassium-40 (40K) into argon-40 (40Ar), which has a half-life of 1.3 billion years.
Key Learning Outcome 2.2. When an atom gains or loses one or more electrons, it is called an ion. Isotopes of an element differ in the number of neutrons they contain, but all have the same chemical properties.