Medical Microbiology
Section 5 Diagnosis and control
35 Passive and non-specific immunotherapy
Introduction
An alternative to vaccination is needed for those who are already infected or are immunodeficient
The most dramatic and successful form of immunotherapy is vaccination, as described in the previous chapter. However, there are some situations where a different approach may be necessary. For instance:
• The patient may already be infected and so a more rapid build-up of immune effector mechanisms than occurs naturally may be needed.
• Alternatively, the patient’s immune system may be inadequate and unable to respond either to the infection or to a vaccine, through immunodeficiency or some especially resistant property of the parasite.
This chapter deals with such situations.
Passive immunization with antibody
Certain diseases are treated by a passive transfer of immunity, which can be life-saving
Before the introduction of antibiotics, acute infectious diseases were often treated by the injection of preformed antibody on the principle that the patient was already ill and it was too late for ‘active’ vaccination. Indeed, the demonstration that immunity to tetanus and diphtheria could be transferred to mice with serum from vaccinated rabbits was a key experiment in the discovery of antibody in the 1890 s. Subsequently, the production of antiserum for the passive treatment of diphtheria, tetanus and pneumococcal pneumonia, and against the toxic effects of streptococci and staphylococci, became an important industry, and generations of horses that had retired from active duty were kept on as the source of ‘immune serum’. The introduction of antitetanus serum in the early months of the First World War reduced the incidence of tetanus dramatically by up to 30-fold (Fig. 35.1).
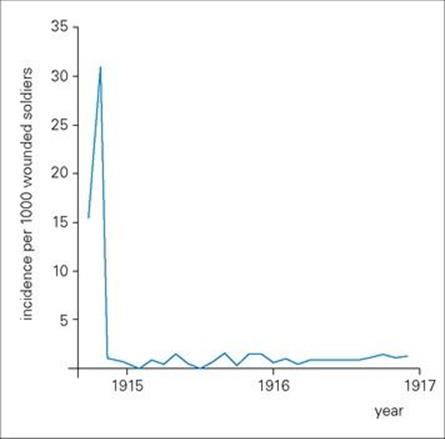
Figure 35.1 Passive immunization significantly reduced the incidence of tetanus in the early months of the First World War. The figure shows the incidence of tetanus per 1000 wounded soldiers in British hospitals during 1914–1916. There was a dramatic fall after the introduction of antitetanus serum in October 1914.
The advent of penicillin and other antibiotics has, of course, changed the picture considerably, and passive immunotherapy is now used for only a select group of diseases (Table 35.1). The serum may be specific or non-specific and of human or animal origin.
Table 35.1 Specific passive immunotherapy with antibody
|
Infection |
Source of antibody |
Indication |
|
Diphtheria |
Human, horse |
|
|
Varicella-zoster |
Human |
Prophylaxis in immunodeficiencies |
|
Gas gangrene |
Horse |
|
|
Rabies |
Human |
Post-exposure (plus vaccine) |
|
Hepatitis B |
Human |
Post-exposure |
|
Hepatitis A |
|
Prophylaxis (travel) |
Although not so commonly used as 50 years ago, passive injections of specific antibody can still be a life-saving treatment.
The use of antiserum raised in animals can cause serum sickness
The use of antiserum raised in horses or rabbits has largely been abandoned because of the complications resulting from the immune response to the antibody, which is of course a foreign protein. These include progressively more rapid elimination (and therefore reduced clinical effectiveness) and, more seriously, serum sickness due to immune complex deposition in, for example, the kidney and skin (see Ch. 17), and even anaphylaxis. These complications can be avoided by using human serum taken during convalescence or following vaccination – to prevent infection after exposure (e.g. rabies) or to minimize its severity (e.g. varicella in immunodeficient children).
Antibody in pooled normal serum can provide protection against infection
With common infections, it can be assumed that most normal people have antibody to the pathogen in their serum. The clearest proof of this is that patients with hypogammaglobulinaemia can be kept free of recurrent infection by regular injections of IgG from pooled normal serum, and that immunodeficient children can be protected against measles in the same way (Box 35.1). Immunoglobulin is prepared from batches of plasma from 1000–6000 healthy donors after screening for hepatitis B and C and HIV. With improvements in methods of preparation, intravenous injection is now preferred to intramuscular injection in most cases. Dosages for this type of therapy range from 100 to 400 mg IgG/kg per month.
![]()
Box 35.1  Indications for Normal Immunoglobulin Therapy
Indications for Normal Immunoglobulin Therapy
Sufficient antibody to protect immunocompromised patients against common infections can be obtained from pooled normal human plasma.
• X-linked agammaglobulinaemia/hypogammaglobulinaemia
• Common variable deficiency
• Wiskott–Aldrich syndrome
• Ataxia telangiectasia
• IgG subclass deficiency with impaired antibody response
• Chronic lymphocytic leukaemia
• Post-bone marrow transplantation (for CMV pneumonitis)
CMV, cytomegalovirus.
![]()
In healthy individuals the probability of contracting hepatitis A in an endemic area is enormously reduced by a single injection of as little as 5 mL of IgG. The immunity conferred by mothers on their newborn infants by placental transfer of IgG and subsequently by colostral IgA (though the latter is not absorbed, but remains in the intestine) is further evidence for the protective effect of relatively small amounts of antibody.
Theoretically, the most effective therapy is provided by one or more monoclonal antibodies specific for a known target antigen
In practice, a mixture of several monoclonal antibodies mimicking just the relevant clones in a polyclonal serum might be required in situations where individual antigens are expressed in low quantities on the microbe or where binding to more than one epitope is required for full effectiveness. We have previously described the derivation of mouse monoclonal antibodies, but a serious complication is that they are highly immunogenic in humans and give rise to human anti-mouse antibodies (HAMA) which accelerate clearance of the monoclonal from the blood and possibly cause hypersensitivity reactions; they also prevent the mouse antibody from reaching its target and, in some cases, block its binding to antigen. Logic points to removal of the xenogeneic (foreign) portions of the monoclonal antibody and their replacement by human Ig structures using recombinant DNA technology. One refined approach is to graft the six complementarity determining regions (CDR) of a high-affinity rodent monoclonal onto a completely human Ig framework without loss of specific reactivity (Fig. 35.2). This is not a trivial exercise, however, and the objective of fusing human B cells to make hybridomas is still appealing, taking into account not only the gross reduction in immunogenicity but also the fact that, within a species, antibodies can be made to subtle differences such as major histocompatibility complex (MHC) polymorphic molecules and tumour-associated antigens on other individuals, whereas xenogeneic responses are more directed to immunodominant structures common to most subjects. Notwithstanding the difficulties in finding good fusion partners, large numbers of human monoclonals have been established.
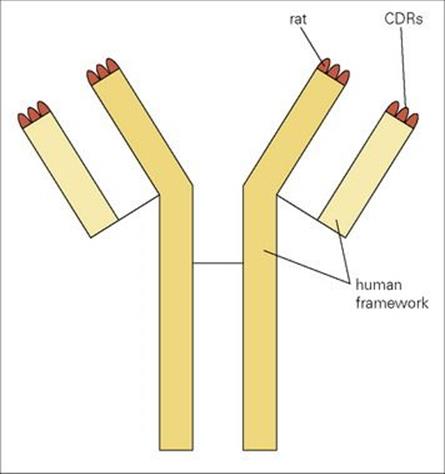
Figure 35.2 Grafting of all six rat complementarity determining regions (CDRs) onto a human Ig framework to create a ‘humanized’ rat monoclonal antibody.
A radically different approach involves the production of transgenic XenoMouse strains in which megabase size unrearranged human Ig H and κ light chain loci have been introduced into mice whose endogenous murine Ig genes have been inactivated. Immunization of these mice yields high-affinity (10− 10–10− 11M) human antibodies which can then be isolated using hybridoma or recombinant approaches. Potent anti-inflammatory (anti-IL-8) and antitumor (anti-epidermal growth factor receptor) therapeutic agents have already been obtained using such mice. There is still a snag in that even human antibodies can provoke anti-idiotype responses; these may have to be circumvented by using engineered antibodies bearing different idiotypes for subsequent injections. Many human monoclonals are being evaluated for clinical use; one can cite IgG anti-RhD for the prevention of rhesus disease of the newborn, and highly potent monoclonals for protection against varicella-zoster, cytomegalovirus, group B streptococci and lipopolysaccharide endotoxins of Gram-negative bacteria.
Not only can the genes for a monoclonal antibody be engineered for expression in bulk in the milk of lactating animals but plants can also be exploited for this purpose, even producing secretory IgA. So-called ‘plantibodies’ have been expressed in bananas, potatoes and tobacco plants. Just imagine a high-tech farmer with one field growing antitetanus toxoid, another antimeningococcal polysaccharide, and so on – the very stuff of science fiction!
Engineering antibodies
Bacteriophage libraries provide an invaluable technology for engineering new antibody fragments
There are other ways around the problems associated with the production of human monoclonals which exploit the wiles of modern molecular biology. Reference has already been made to the ‘humanizing’ of rodent antibodies, but an important new strategy based upon bacteriophage expression and selection has achieved a prominent position. In essence, mRNA, preferably from primed human B cells, is converted to cDNA, and the antibody genes, or fragments therefrom, are expanded by the polymerase chain reaction (PCR). Single constructs are then made in which the light and heavy chain genes are allowed to combine randomly as Fab or single chain Fv (scFv) fragments in tandem with the bacteriophage coat protein gene (Fig. 35.3). This combinatorial library encodes a huge repertoire of antibody fragments expressed as fusion proteins with a filamentous coat protein on the bacteriophage surface. The extremely high number of phages produced by Escherichia coli infection can now be panned on solid-phase antigen to select those bearing the highest-affinity antibodies attached to their surface (Fig. 35.3). Because the genes which encode these highest-affinity antibodies are already present within the selected phage, they can readily be cloned and the antibody fragment expressed in bulk.
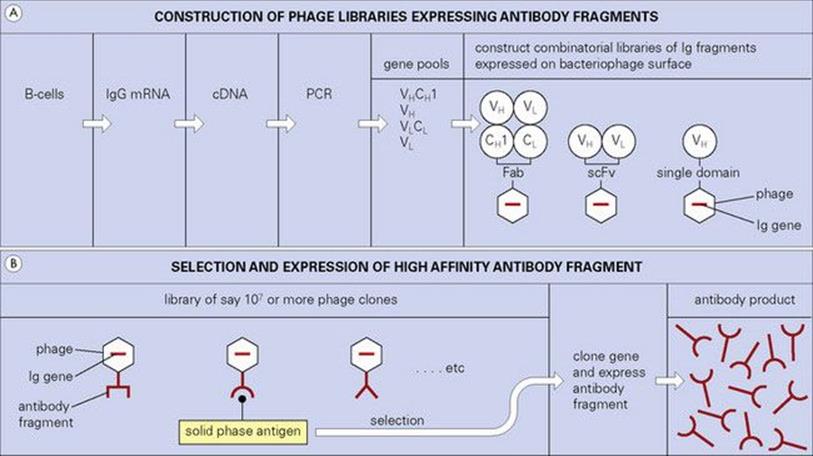
Figure 35.3 Pools of genes encoding Ig domains derived from IgG mRNA are randomly combined and expressed either as Fab or single chain Fv (scFv) fragments on the surface of the bacteriophage. Libraries expressing single domains of the heavy chain variable region (VH) (human or llama, usually) can also be constructed. Phage clones containing genes encoding high-affinity antibody fragments can be selected from these extremely large libraries using solid-phage antigen. The appropriate Ig genes can then be cloned and expressed in suitable vectors to produce abundant antibody fragments.
It should be recognized that this selection procedure has an enormous advantage over techniques which employ screening, because the number of phages which can be examined is several logs higher. Although a ‘test-tube’ operation, this approach to the generation of specific antibodies does resemble the affinity maturation of the immune response in vivo in the sense that antigen is the determining factor in selecting out the highest-affinity responders. In order to increase the affinities of antibodies produced by these techniques, antigen can be used to select higher-affinity mutants produced by random mutagenesis (or even more effectively by site-directed replacements at mutational hot spots), again mimicking the natural immune response which involves random mutation and antigen selection.
Single domain variable region fragments have several advantages
Phage libraries have been created which express just single heavy or light chain variable region domains (VH or VL dAbs). When selected from large naive human phage libraries and fine-tuned by random mutation and further selection, dAbs of surprisingly high affinity, sometimes in the low nanomolar range, can be obtained, clearly without the need for prior immunization. Camelids are immunologically curious in that one half of their antibodies are conventionally composed of heavy and light chains but the other half are just heavy chains, albeit with unusual CDRs which can subserve high-affinity interactions with antigen. Thus a parallel technology has developed in which high-affinity VHH (variable domains from heavy chain antibodies) have been selected from immunized llamas.
Both human and llama VH dAbs have several advantages. They are easy to engineer in bulk cheaply, they can readily be custom-tailored by molecular biological manipulations, they are small and robust in their ability to withstand variations in temperature and acidity, making them relatively insensitive to environmental conditions and the need for refrigeration, and permitting their use for oral therapy and for repeated affinity chromatographic purification of antigens. Another advantage is their low immunogenicity.
Antibody fragments lacking the Fc structures required for secondary activity obviously will not provide protection where complement fixation, phagocytic uptake or extracellular killing is required to eliminate a pathogen. Where they are effective is in blocking cognate enzyme–substrate, hormone or toxin–receptor and microbial addressin–epithelial cell receptor interactions. The latter situation is particularly relevant to mucosal infections where specific adherence to a cognate epithelial receptor is an essential initial step in the infectious process. Initial studies have shown efficacy of dAbs in preventing experimental rotavirus infection and vaginal candidiasis (Fig. 35.4).
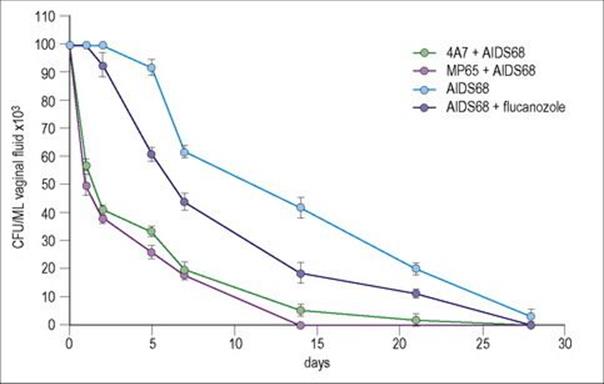
Figure 35.4 Protective activity of an anti-Sap2 (4A7) and an anti-MP65 human variable region single domain antibody (dAb) against rat vaginal infection by a C. albicans fluconazole-resistant strain (AIDS 68). Five rats per group were used. Each animal was administered intravaginally 20 μg of each dAb 30 min before intravaginal challenge with 107 fungal cells. Fluconazole was used as a single intravaginal dose of 50 μg, 30 min before challenge. Irrelevant dAbs were not protective. Efficacy in protection against infection paralleled the ability of the dAb to inhibit the adherence of Candida to cultures of epithelial cells.
(Courtesy of F. de Bernadis, and A. Cassone et al.).
Non-specific cellular immunostimulation
Cytokines and other molecular mediators stimulate the immune system
The demonstration by William Coley almost one century ago that crude extracts of bacteria could induce remission and sometimes cure cancers indicated the extent to which the immune system can be non-specifically ‘overstimulated’, with potentially beneficial results. Until recently, many of the compounds used in this way have been of microbial origin, but current interest is directed mainly at cytokines and other molecular mediators, on the principle that their induction was probably the basis of action of the older crude materials (Box 35.2).
![]()
Box 35.2  Non-specific Immunostimulators
Non-specific Immunostimulators
A variety of foreign and endogenous materials have been used in an attempt to raise the general level of immunologic competence.
Microbial
• Coley’s toxin (filtered cultures of Streptococci and Serratia marcescens used against tumours)
• BCG (bacillus Calmette–Guérin)
• Corynebacterium parvum
• Endotoxin (lipopolysaccharide)
• Streptococcal-derived OK432
Endogenous
• Thymus factors and hormones
• Cytokines
![]()
Most of the applications of this type of immunostimulation have been in the tumour field, but some infectious diseases respond to treatment with cytokines (Table 35.2). Foremost among these are the interferons (IFNs), notably IFNα, which is effective in a number of virus infections, though less than might have been predicted from the importance of its normal role in inhibiting viral replication. IFNγ has recently been found to benefit many cases of chronic granulomatous disease (CGD), though the mechanism is unclear. The unpleasant side effects of high-dose therapy with interleukins or IFNs restrict their casual use (Table 35.3).
Table 35.2 Potentially therapeutic cytokines and their antagonists
|
IFNα, IFNβ |
Hepatitis B (chronic) |
|
Hepatitis C |
|
|
Herpes zoster |
|
|
Papillomavirus |
|
|
Rhinovirus (prophylactic only) |
|
|
HIV |
|
|
Warts |
|
|
IFNγ |
Lepromatous leprosy |
|
Leishmaniasis |
|
|
Toxoplasmosis (brain) |
|
|
Chronic granulomatous disease |
|
|
IL-2 |
Leprosy (local treatment to skin lesions) |
|
IL-10, TGFβ |
Septic shock |
|
CSFs |
Bacterial infection due to neutropenia in irradiated patients |
|
Anti-TNF |
Septic shock |
|
IL-1 receptor antagonist |
Septic shock |
Cytokines are increasingly used to improve immunity to infection as well as for some cancers and haematologic disorders. CSFs, colony stimulating factors; IFN, interferon; IL, interleukin; TGF, transforming growth factor; TNF, tumour necrosis factor
Table 35.3 Some common side effects of cytokine therapy
|
Interferons |
Fever |
|
IL-2 |
Vascular leak syndrome |
Treatment with cytokines, especially if prolonged, can lead to serious side effects. IL, interleukin.
There is an interesting ‘grey area’ where immunostimulation and nutrition overlap
It has been claimed for many years that transfer factor (TF), a dialysed extract of peripheral leukocytes from normal patients, will restore T-cell responses in unresponsive recipients, and some dramatic cures (e.g. of chronic mucocutaneous candidiasis) have been reported. Whether this restoration is antigen specific or non-specific has been the subject of great controversy, and in the absence of proper molecular characterization, TF is no longer regarded as an orthodox treatment.
Equally unorthodox, but attracting increasing attention, are a variety of plant products (e.g. saponins, ginseng, Chinese herbal remedies). These substances appear to improve resistance to infection and in some cases also act as adjuvants when combined with vaccines, but the complexity and variability of the extracts makes the active components difficult to track down.
Correction of host immunodeficiency
Antibody defects are the easiest to treat
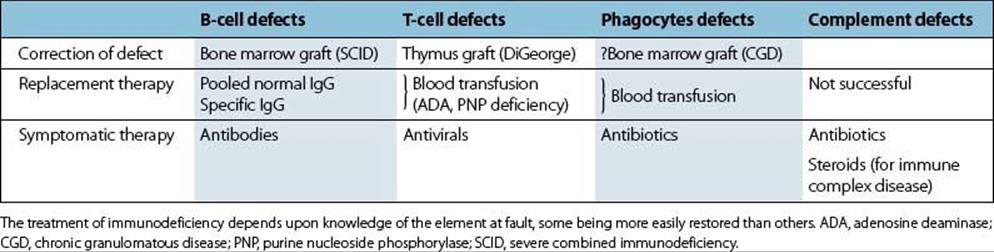
This subject is discussed in more detail in Chapter 30, and will only be briefly summarized here:
• Antibody defects are the easiest to treat, since immunoglobulin can be transferred and has a reasonably long half-life (about 3 weeks for IgG).
• Treatment of T-cell defects is more difficult, though thymus or bone marrow grafting has been tried in certain cases with some success (Table 35.4).
• Phagocytic defects are the most difficult to correct, and in practice antibiotics remain the mainstay of therapy, though the future may lie in gene replacement.
Table 35.4 Treatment of immunodeficiency: an overview
Gene defects have recently been identified in certain serious immunodeficiency diseases including hyper-IgM syndrome, CGD and Bruton’s agammaglobulinaemia.
Probiotics
Probiotics are dietary supplements containing potentially beneficial bacteria or yeast of which lactic acid bacteria are the most common microbes used. The gut flora can be thrown out of balance by a wide range of circumstances including the use of antibiotics or other drugs, excess alcohol, stress, disease, exposure to toxic substances or even the use of antibacterial soap. In cases like these, the ‘friendly’ bacteria, which work well with our bodies, may decrease in number, so allowing harmful competitors to thrive to the detriment of our health. Probiotic bacterial cultures are intended to assist the body’s naturally occurring flora within the digestive tract to re-establish themselves. They are sometimes recommended by doctors, and more frequently by nutritionists, after a course of antibiotics or as part of the treatment for candidiasis. Many probiotics are present in natural sources such as yoghurt, commonly used microbes being Lactobacillus acidophilus and Bifidobacterium bifidum.
A range of potentially beneficial medicinal uses for probiotics have been explored and these include managing lactose intolerance, prevention of colon cancer, cholesterol lowering, improving immune function and preventing infections, and reducing inflammation. It is also possible to increase and maintain a healthy gut flora by increasing the amounts of prebiotics in the diet, such as inulin, raw oats and unrefined wheat; a combination of the two should prove to be synergistic since prebiotics are only effective in the large intestine, whereas probiotics exert their influence in the small bowel. It must be said that robust evidence to support these claims is still being acquired.
![]()
 Key Facts
Key Facts
• Transfer of normal pooled IgG is the most widely practised type of passive immunotherapy and is used to treat most forms of antibody deficiency.
• Specific antibodies can be used for certain defined conditions. Such antibodies can be produced as mouse or human monoclonals, as rodent complementarity determining regions (CDRs) grafted onto a human Ig framework.
• Antibodies can be engineered for expression in bulk in conventional vectors in vitro, or in vivo in the milk of lactating animals or in plants.
• Fab, single chain Fv (scFv) or heavy chain variable region domain fragments can be selected by antigen from expression libraries of bacteriophages bearing the antibody fragments as a surface protein.
• These fragments are effective in blocking cognate interactions such as microbial addressin adherence to mucosal epithelial cells as a precursor to invasion. Single domain human and llama variable region antibody fragments are robust and have several advantages.
• Non-specific stimulation of T-cell-mediated immunity is still experimental, but cytokines show some promise, particularly IFN for viral infections.
![]()