Lippincott’s Illustrated Reviews: Biochemistr, Sixth Edition (2014)
UNIT III: Lipid Metabolism
Chapter 16. Fatty Acid, Ketone Body, and Triacylglycerol Metabolism
I. OVERVIEW
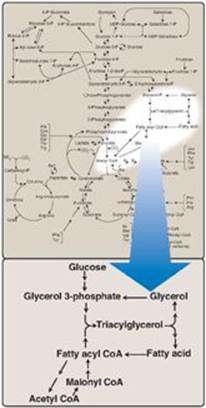
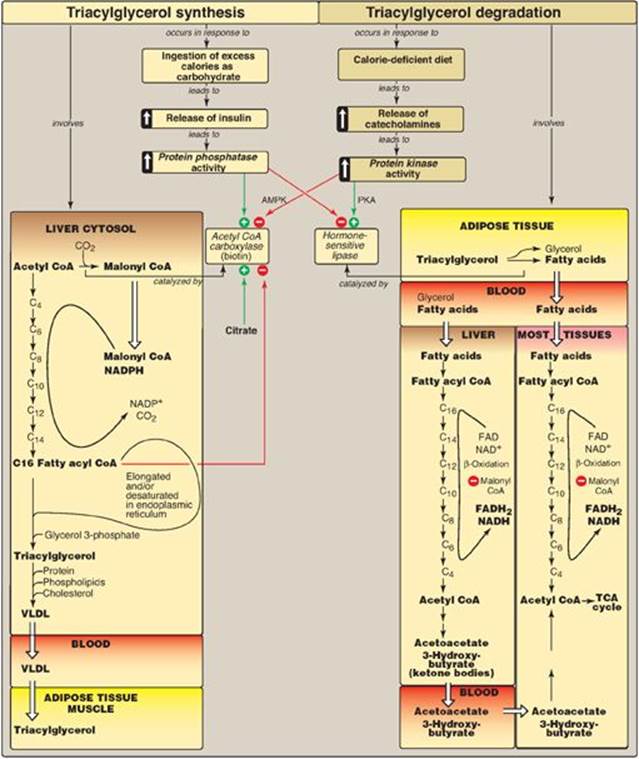
Fatty acids exist “free” in the body (that is, they are unesterified) and as fatty acyl esters in more complex molecules such as triacylglycerols (TAGs). Low levels of free fatty acids (FFAs) occur in all tissues, but substantial amounts can sometimes be found in the plasma, particularly during fasting. Plasma FFAs (transported on serum albumin) are in route from their point of origin (TAG of adipose tissue or circulating lipoproteins) to their site of consumption (most tissues). FFAs can be oxidized by many tissues, particularly liver and muscle, to provide energy and, in liver, to provide the substrate for ketone body synthesis. Fatty acids are also structural components of membrane lipids, such as phospholipids and glycolipids (see p. 201). Fatty acids attached to certain proteins enhance the ability of those proteins to associate with membranes (see p. 206). Fatty acids are also precursors of the hormone-like prostaglandins (see p. 213). Esterified fatty acids, in the form of TAGs stored in white adipose tissue (WAT), serve as the major energy reserve of the body. Alterations in fatty acid metabolism are associated with obesity and diabetes. Figure 16.1 illustrates the metabolic pathways of fatty acid synthesis and degradation and their relationship to carbohydrate metabolism.
Figure 16.1 Triacylglycerol synthesis and degradation. CoA = coenzyme A.
II. STRUCTURE OF FATTY ACIDS
A fatty acid consists of a hydrophobic hydrocarbon chain with a terminal carboxyl group that has a pKa of about 4.8 (Figure 16.2). At physiologic pH, the terminal carboxyl group (–COOH) ionizes, becoming –COO–. [Note: When the pH is above the pK, the deprotonated form predominates (see p.7).] This anionic group has an affinity for water, giving the fatty acid its amphipathic nature (having both a hydrophilic and a hydrophobic region). However, for long-chain fatty acids (LCFAs), the hydrophobic portion is predominant. These molecules are highly water insoluble and must be transported in the circulation in association with protein. More than 90% of the fatty acids found in plasma are in the form of fatty acid esters (primarily TAG, cholesteryl esters, and phospholipids) contained in circulating lipoprotein particles (see p. 227). Unesterified (free) fatty acids are transported in the circulation in association with albumin, the most abundant protein in serum.
Figure 16.2 Structure of a fatty acid.

A. Saturation of fatty acids
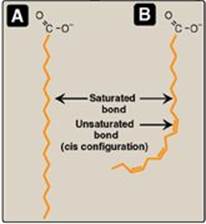
Fatty acid chains may contain no double bonds (that is, be saturated) or contain one or more double bonds (that is, be mono- or polyunsaturated). In humans, the majority are saturated or monounsaturated. When double bonds are present, they are nearly always in the cis rather than in the trans configuration. (See p. 363 for a discussion of the dietary occurrence of cis and trans unsaturated fatty acids.) The introduction of a cis double bond causes the fatty acid to bend or “kink” at that position (Figure 16.3). If the fatty acid has two or more double bonds, they are always spaced at three-carbon intervals. [Note: In general, addition of double bonds decreases the melting temperature (Tm) of a fatty acid, whereas increasing the chain length increases the Tm. Because membrane lipids typically contain LCFAs, the presence of double bonds in some fatty acids helps maintain the fluid nature of those lipids.]
Figure 16.3 A saturated (A) and an unsaturated (B) fatty acid. Orange denotes hydrophobic portions of the molecules. [Note: Cis double bonds cause a fatty acid to “kink.”].
B. Chain lengths of fatty acids and positions of double bonds
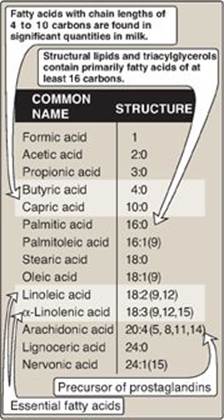
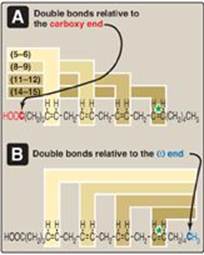
The common names and structures of some fatty acids of physiologic importance are listed in Figure 16.4. In humans, fatty acids with an even number of carbon atoms (16, 18, or 20) predominate, with longer fatty acids (over 22 carbons) being found in the brain. The carbon atoms are numbered, beginning with the carboxyl carbon as carbon 1. The number before the colon indicates the number of carbons in the chain, and those after the colon indicate the numbers and positions (relative to the carboxyl end) of double bonds. For example, as denoted in Figure 16.4, arachidonic acid, 20:4(5,8,11,14), is 20 carbons long and has 4 double bonds (between carbons 5–6, 8–9, 11–12, and 14–15). [Note: Carbon 2, the carbon to which the carboxyl group is attached, is also called the α-carbon, carbon 3 is the β-carbon, and carbon 4 is the γ-carbon. The carbon of the terminal methyl group is called the ω-carbon regardless of the chain length.] The double bonds in a fatty acid can also be denoted relative to the ω (or methyl) end of the chain. Arachidonic acid is referred to as an ω-6 fatty acid acid (also an n-6 fatty acid, Figure 16.5A) because the terminal double bond is six bonds in from the ω end (Figure 16.5B). Another ω-6 fatty acid is the essential linoleic acid 18:2(9,12). In contrast, α-linolenic acid, 18:3(9,12,15), is an essential ω-3 fatty acid. (See p. 363 for a discussion of the nutritional significance of ω-3 and ω-6 fatty acids.)
Figure 16.4 Some fatty acids of physiologic importance. [Note: If a fatty acid has 2–4 carbons, it is considered short; if 6–12, medium; if 14–20, long; and if 22 or more, very long.].
C. Essential fatty acids
Linoleic acid, the precursor of ω-6 arachidonic acid, which is the substrate for prostaglandin synthesis (see p. 213), and α-linolenic acid, the precursor of ω-3 fatty acids that are important for growth and development, are dietary essentials in humans because we lack the enzymes needed to synthesize them. Plants provide us with these essential fatty acids. [Note: Arachidonic acid becomes essential if linoleic acid is deficient in the diet.]
Essential fatty acid deficiency (rare) can result in a dry, scaly dermatitis as a result of an inability to synthesize molecules that provide the water barrier in skin (see p. 206).
III. DE NOVO SYNTHESIS OF FATTY ACIDS
A large proportion of the fatty acids used by the body is supplied by the diet. Carbohydrates and protein obtained from the diet in excess of the body’s needs for these compounds can be converted to fatty acids, which are stored as TAGs. (See p. 321 for a discussion of the metabolism of dietary nutrients in the well-fed state.) In adult humans, fatty acid synthesis occurs primarily in the liver and lactating mammary glands and, to a lesser extent, in adipose tissue. This cytosolic process incorporates carbons from acetyl coenzyme A (CoA) into the growing fatty acid chain, using adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide phosphate (NADPH).
Figure 16.5 Arachidonic acid, illustrating the position of the double bonds. Arachidonic acid, 20:4(5,8,11,14) is an n-6 fatty acid because the double bond furthest from the carboxy end (carbon 1) is 14 carbons from that end: 20 - 14 = 6. It is also referred to as an ω-6 fatty acid because the terminal double bond is six bonds in from the ω end. Thus, the “ω” and “n” designations are equivalent (see *).
A. Production of cytosolic acetyl coenzyme A
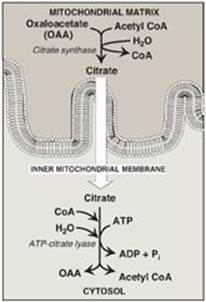
The first step in de novo fatty acid synthesis is the transfer of acetate units from mitochondrial acetyl CoA to the cytosol. Mitochondrial acetyl CoA is produced by the oxidation of pyruvate (see p. 109) and by the catabolism of certain amino acids (see p. 266). The CoA portion of acetyl CoA, however, cannot cross the inner mitochondrial membrane, and only the acetyl portion enters the cytosol. It does so as part of citrate produced by the condensation of acetyl CoA with oxaloacetate (OAA) by citrate synthase (Figure 16.6). [Note: The translocation of citrate to the cytosol occurs when the mitochondrial citrate concentration is high. This is observed when isocitrate dehydrogenase of the citric acid cycle is inhibited by the presence of large amounts of ATP, causing citrate and isocitrate to accumulate (see p. 112). Therefore, cytosolic citrate may be viewed as a high-energy signal. Because a large amount of ATP is needed for fatty acid synthesis, the increase in both ATP and citrate enhances this pathway.]
B. Carboxylation of acetyl coenzyme A to malonyl coenzyme A
The energy for the carbon-to-carbon condensations in fatty acid synthesis is supplied by the process of carboxylation followed by decarboxylation of acyl groups in the cytosol. The carboxylation of acetyl CoA to form malonyl CoA is catalyzed by acetyl CoA carboxylase (ACC) (Figure 16.7), and requires CO2 and ATP. The coenzyme is the vitamin biotin, which is covalently bound to a lysyl residue of the carboxylase (see Figure 28.16, p. 381). ACCcarboxylates the bound biotin, which transfers the activated carboxyl group to acetyl CoA.
Figure 16.6 Production of cytosolic acetyl coenzyme A (CoA). Citrate is transported by the tricarboxylate transporter system. ADP = adenosine monophosphate; Pi = inorganic phosphate.
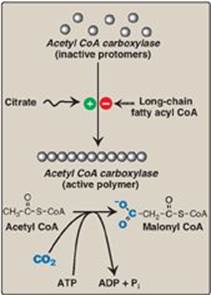
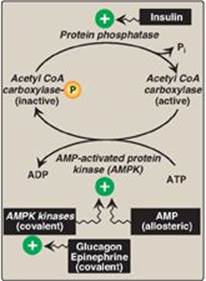
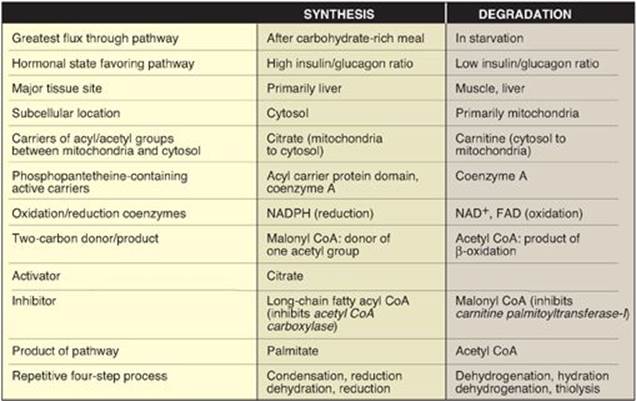
1. Short-term regulation of acetyl coenzyme A carboxylase: This carboxylation is both the rate-limiting and the regulated step in fatty acid synthesis (see Figure 16.7). The inactive form of ACC is a protomer. The enzyme undergoes allosteric activation by citrate, which causes protomers to polymerize, and allosteric inactivation by long-chain fatty acyl CoA (the end product of the pathway), which causes depolymerization. A second mechanism of short-term regulation is by reversible phosphorylation. Adenosine monophosphate–activated protein kinase (AMPK) phosphorylates and inactivates ACC. AMPK itself is allosterically activated by AMP and covalently activated by phosphorylation via several kinases. At least one of these AMPK kinases is activated by cAMP-dependent protein kinase A (PKA). Thus, in the presence of counterregulatory hormones, such as epinephrine and glucagon, ACC is phosphorylated and, thereby, inactivated (Figure 16.8). In the presence of insulin, ACC is dephosphorylated and, thereby, activated. [Note: This is analogous to the regulation of glycogen synthase (see p. 131).]
2. Long-term regulation of acetyl coenzyme A carboxylase: Prolonged consumption of a diet containing excess calories (particularly high-calorie, high-carbohydrate diets) causes an increase in ACC synthesis, thereby increasing fatty acid synthesis. Conversely, a low-calorie or a high-fat diet causes a reduction in fatty acid synthesis by decreasing ACC synthesis. [Note: Synthesis of the carboxylase is upregulated by insulin via a sterol regulatory element–binding protein, SREBP-1. The function and regulation of SREBPs are described on p. 222. Fatty acid synthase (see below) is similarly regulated by diet and SREBP-1.] Metformin, used in the treatment of type 2 diabetes, lowers serum TAG through activation of AMPK, resulting in inhibition of ACC activity (by phosphorylation) and inhibition of ACC and fatty acid synthase expression (by decreasing SREBP-1). Metformin also lowers blood glucose by increasing AMPK-mediated uptake of glucose by muscle.
Figure 16.7 Allosteric regulation of malonyl coenzyme A (CoA) synthesis by acetyl CoA carboxylase. The carboxyl group contributed by dissolved CO2 is shown in blue. Pi = inorganic phosphate; ADP = adenosine diphosphate.
C. Fatty acid synthase: a multifunctional enzyme in eukaryotes
The remaining series of reactions of fatty acid synthesis in eukaryotes is catalyzed by the multifunctional, dimeric enzyme, fatty acid synthase (FAS). Each FAS monomer is a multicatalytic polypeptide with seven different enzymic domains plus a domain that covalently binds a molecule of 4ʹ-phosphopantetheine. [Note: 4ʹ-Phosphopantetheine, a derivative of the vitamin pantothenic acid (see p. 381), carries acyl units on its terminal thiol (–SH) group during fatty acid synthesis. It also is a component of CoA.] In prokaryotes, FAS is a multienzyme complex, and the 4ʹ-phosphopantetheine domain is a separate protein, referred to as the acyl carrier protein (ACP). ACP is used to refer to the phosphopantetheine-containing domain of eukaryotic FAS. The reaction numbers in brackets below refer to Figure 16.9.
[1] An acetyl group is transferred from acetyl CoA to the –SH group of the ACP. Domain: Acetyl CoA-ACP acetyltransacylase.
[2] Next, this two-carbon fragment is transferred to a temporary holding site, the thiol group of a cysteine residue on the enzyme.
[3] The now-vacant ACP accepts a three-carbon malonyl group from malonyl CoA. Domain: Malonyl CoA-ACP transacylase.
[4] The acetyl group on the cysteine residue condenses with the malonyl group on ACP as the CO2 originally added by acetyl CoA carboxylase is released. The result is a four-carbon unit attached to the ACP domain. The loss of free energy from the decarboxylation drives the reaction. Domain: 3-Ketoacyl-ACP synthase, also known as “condensing enzyme.”
Figure 16.8 Covalent regulation (phosphorylation) of acetyl CoA carboxylase by AMPK, which itself is regulated both covalently and allosterically. CoA = coenzyme A; ADP = adenosine diphosphate; ![]() = phosphate; Pi = inorganic phosphate; AMP = adenosine monophosphate.
= phosphate; Pi = inorganic phosphate; AMP = adenosine monophosphate.
The next three reactions convert the 3-ketoacyl group to the corresponding saturated acyl group by a pair of NADPH-requiring reductions and a dehydration step.
[5] The keto group is reduced to an alcohol. Domain: 3-Ketoacyl-ACP reductase.
Figure 16.9 Synthesis of palmitate (16:0) by multifunctional fatty acid synthase (FAS). [Note: Numbers in brackets correspond to bracketed numbers in the text. A second repetition of the steps is indicated by numbers with an asterisk (*).
Carbons provided directly by acetyl coenzyme A (CoA) are shown in red.] Cys = cysteine; ACP = acyl carrier protein domain; NADP(H) = nicotinamide adenine dinucleotide phosphate.
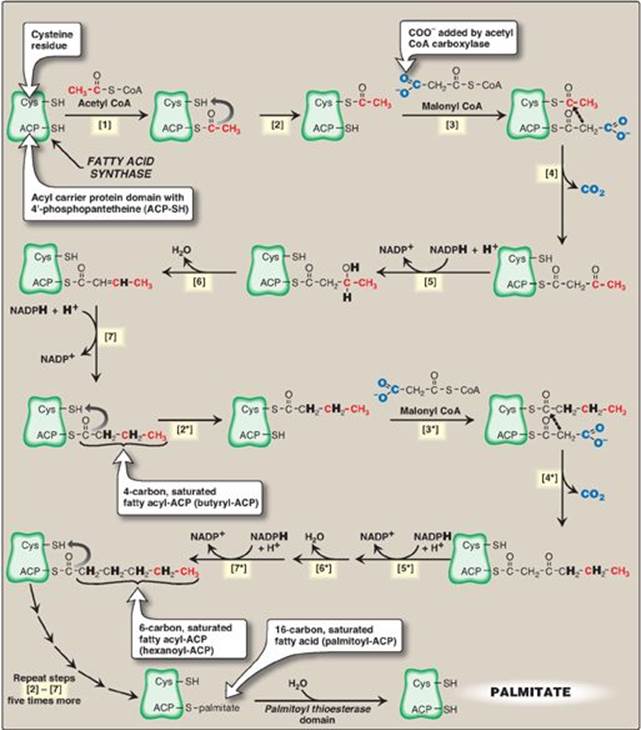
[6] A molecule of water is removed, creating a double bond between carbons 2 and 3 (the α- and β-carbons). Domain: 3-Hydroxyacyl-ACP dehydratase.
[7] The double bond is reduced. Domain: Enoyl-ACP reductase.
The result of these seven steps is production of a four-carbon compound (butyryl) whose three terminal carbons are fully saturated, and which remains attached to the ACP domain. These seven steps are repeated, beginning with the transfer of the butyryl chain from the ACP to the cysteine residue [2*], the attachment of a molecule of malonate to the ACP [3*], and the condensation of the two molecules liberating CO2 [4*]. The carbonyl group at the β-carbon (carbon 3, the third carbon from the sulfur) is then reduced [5*], dehydrated [6*], and reduced [7*], generating hexanoyl-ACP. This cycle of reactions is repeated five more times, each time incorporating a two-carbon unit (derived from malonyl CoA) into the growing fatty acid chain at the carboxyl end. When the fatty acid reaches a length of 16 carbons, the synthetic process is terminated with palmitoyl-S-ACP. [Note: Shorter-length fatty acids are important end products in the lactating mammary gland.] Palmitoyl thioesterase, the final catalytic activity of FAS, cleaves the thioester bond, releasing a fully saturated molecule of palmitate (16:0). [Note: All the carbons in palmitic acid have passed through malonyl CoA except the two donated by the original acetyl CoA, which are found at the methyl (ω) end of the fatty acid. This underscores the rate-limiting nature of the ACC reaction.]
Figure 16.10 Cytosolic conversion of oxaloacetate to pyruvate with the generation of nicotinamide adenine dinucleotide phosphate (NADPH). [Note: The pentose phosphate pathway is also a source of NADPH.] NAD(H) = nicotinamide adenine dinucleotide.
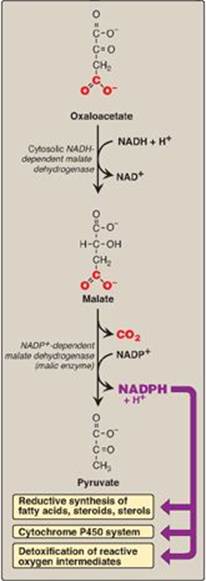
D. Major sources of the reductant required for fatty acid synthesis
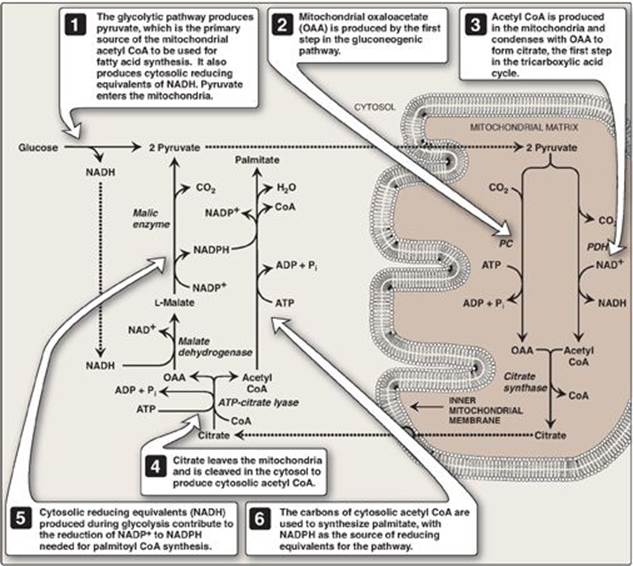
The pentose phosphate pathway (see p. 145) is a major supplier of NADPH, the reducatant required for fatty acid synthesis. Two NADPH are produced for each molecule of glucose that enters this pathway. The cytosolic conversion of malate to pyruvate, in which malate is oxidized and decarboxylated by cytosolic malic enzyme (NADP+-dependent malate dehydrogenase), also produces cytosolic NADPH (and CO2) as shown in Figure 16.10. [Note: Malate can arise from the reduction of OAA by cytosolic NADH-dependent malate dehydrogenase (see Figure 16.10). One source of the cytosolic NADH required for this reaction is that produced during glycolysis (see p. 101). OAA, in turn, can arise from citrate. Recall from Figure 16.6 that citrate, formed from OAA and acetyl CoA by citrate synthase, was shown to move from the mitochondria into the cytosol, where it is cleaved into acetyl CoA and OAA by ATP-citrate lyase.] A summary of the interrelationship between glucose metabolism and palmitate synthesis is shown in Figure 16.11.
E. Further elongation of fatty acid chains
Although palmitate, a 16-carbon, fully saturated LCFA (16:0), is the primary end product of fatty acid synthase activity, it can be further elongated by the addition of two-carbon units to the carboxylate end in the smooth endoplasmic reticulum (SER). Elongation requires a system of separate enzymes rather than a multifunctional enzyme. Malonyl CoA is the two-carbon donor, and NADPH supplies the electrons. The brain has additional elongation capabilities, allowing it to produce the very-long-chain fatty acids ([VLCFAs] over 22 carbons) that are required for synthesis of brain lipids.
Figure 16.11 Interrelationship between glucose metabolism and palmitate synthesis. CoA = coenzyme A; NAD(H) = nicotinamide adenine nucleotide; NADP(H) =nicotinamide adenine dinucleotide phosphate; ADP = adenosine diphosphate; Pi = inorganic phosphate; PC = pyruvate carboxylase; PDH = pyruvate dehydrogenase.
F. Desaturation of fatty acid chains
Enzymes (desaturases) also present in the SER are responsible for desaturating LCFAs (that is, adding cis double bonds). The desaturation reactions require O2, NADH, cytochrome b5, and its FAD-linked reductase. The fatty acid and the NADH get oxidized as the O2 gets reduced to H2O. The first double bond is typically inserted between carbons 9 and 10, producing primarily oleic acid, 18:1(9), and small amounts of palmitoleic acid, 16:1(9). A variety of polyunsaturated fatty acids can be made through additional desaturation combined with elongation.
Humans have carbon 9, 6, 5, and 4 desaturases but lack the ability to introduce double bonds from carbon 10 to the ω end of the chain. This is the basis for the nutritional essentiality of the polyunsaturated acids ω-6 linoleic and ω-3 linolenic.
G. Storage of fatty acids as components of triacylglycerols
Mono-, di-, and triacylglycerols consist of one, two, or three molecules of fatty acid esterified to a molecule of glycerol. Fatty acids are esterified through their carboxyl groups, resulting in a loss of negative charge and formation of “neutral fat.” [Note: If a species of acylglycerol is solid at room temperature, it is called a fat, whereas if it is liquid, it is called an oil.]
1. Structure: The three fatty acids esterified to a glycerol molecule to form a TAG are usually not of the same type. The fatty acid on carbon 1 is typically saturated, that on carbon 2 is typically unsaturated, and that on carbon 3 can be either. Recall that the presence of the unsaturated fatty acid(s) decrease(s) the Tm of the lipid. An example of a TAG molecule is shown in Figure 16.12.
Figure 16.12 A triacylglycerol with an unsaturated fatty acid on carbon 2. Orange denotes the hydrophobic portions of the molecule.
2. Storage: Because TAGs are only slightly soluble in water and cannot form stable micelles by themselves, they coalesce within white adipocytes to form large oily droplets that are nearly anhydrous. These cytosolic lipid droplets are the major energy reserve of the body. [Note: TAGs stored in brown adipocytes serve as a source of heat through nonshivering thermogenesis (see p. 78).]
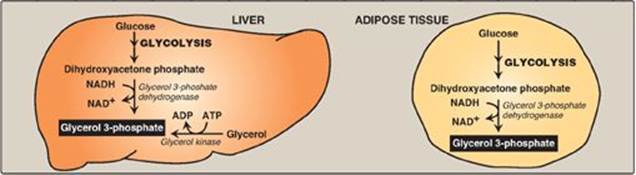
3. Synthesis of glycerol 3-phosphate: Glycerol 3-phosphate is the initial acceptor of fatty acids during TAG synthesis. There are two pathways for its production (Figure 16.13). In both liver (the primary site of TAG synthesis) and adipose tissue, glycerol 3-phosphate can be produced from glucose, using first the reactions of the glycolytic pathway to produce dihydroxyacetone phosphate ([DHAP], see p. 101). DHAP is reduced by glycerol 3-phosphate dehydrogenase to glycerol 3-phosphate. A second pathway found in the liver, but not in adipose tissue, uses glycerol kinase to convert free glycerol to glycerol phosphate (see Figure 16.13). [Note: The glucose transporter in adipocytes (GLUT-4) is insulin dependent (see p. 312). Thus, when plasma glucose (and, therefore, plasma insulin) levels are low, adipocytes have only a limited ability to synthesize glycerol phosphate and cannot produce TAG de novo.]
Figure 16.13 Pathways for production of glycerol 3-phosphate in liver and adipose tissue. [Note: Glycerol 3-phosphate can also be generated by glyceroneogenesis.] NAD(H) = nicotinamide adenine dinucleotide; ADP = adenosine diphosphate.
4. Activation of a free fatty acid: A fatty acid must be converted to its activated form (bound to CoA) before it can participate in metabolic processes such as TAG synthesis. This reaction, illustrated in Figure 15.6, is catalyzed by a family of fatty acyl CoA synthetases (thiokinases).
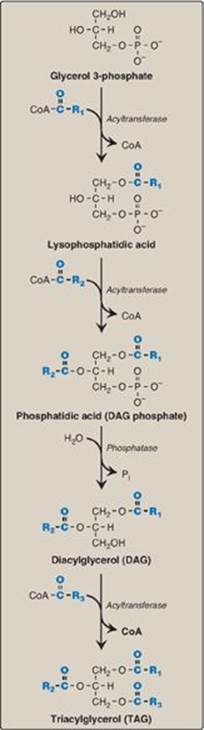
5. Synthesis of triacylglycerol from glycerol 3-phosphate and fatty acyl coenzyme As: This pathway involves four reactions, shown in Figure 16.14. These include the sequential addition of two fatty acids from fatty acyl CoAs, the removal of phosphate, and the addition of the third fatty acid.
H. Different fates of triacylglycerol in liver and adipose tissue
In WAT, TAG is stored in a nearly anhydrous form as fat droplets in the cytosol of the cells. It serves as “depot fat,” ready for mobilization when the body requires it for fuel. Little TAG is stored in healthy liver. Instead, most is exported, packaged with other lipids and apolipoproteins to form lipoprotein particles called very-low-density lipoproteins (VLDLs). Nascent VLDLs are secreted directly into the blood where they mature and function to deliver the endogenously derived lipids to the peripheral tissues. [Note: Recall from Chapter 15 that chylomicrons carry dietary (exogenously derived) lipids.] Plasma lipoproteins are discussed in Chapter 18.
Figure 16.14 Synthesis of TAG. R1-R3 = activated fatty acids. CoA = coenzyme A; Pi = inorganic phosphate.
IV. MOBILIZATION OF STORED FATS AND OXIDATION OF FATTY ACIDS
Fatty acids stored in WAT, in the form of neutral TAG, serve as the body’s major fuel storage reserve. TAGs provide concentrated stores of metabolic energy because they are highly reduced and largely anhydrous. The yield from the complete oxidation of fatty acids to CO2 and H2O is 9 kcal/g fat (as compared to 4 kcal/g protein or carbohydrate, see Figure 27.5 on p. 359).
A. Release of fatty acids from fat
The mobilization of stored fat requires the hydrolytic release of fatty acids and glycerol from their TAG form. This process of lipolysis is achieved by lipases. It is initiated by adipose triglyceride lipase (ATGL), which generates a diacylglycerol that is the preferred substrate for hormone-sensitive lipase (HSL). The monoacylglycerol (MAG) product of HSL is acted upon by MAG lipase.
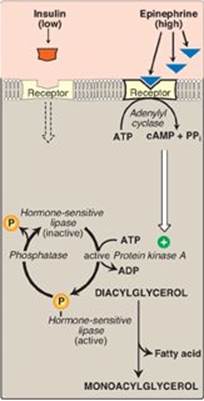
1. Regulation of hormone-sensitive lipase: HSL is active when phosphorylated by PKA, a 3′,5′-cyclic AMP(cAMP)–dependent protein kinase. cAMP is produced in the adipocyte when catecholamines (such as epinephrine) bind to cell membrane β-adrenergic receptors and activate adenylyl cyclase (Figure 16.15). The process is similar to that of the activation of glycogen phosphorylase (see Figure 11.9). [Note: Because ACC is inhibited by hormone-directed phosphorylation, when the cAMP-mediated cascade is activated (see Figure 16.8), fatty acid synthesis is turned off and TAG degradation is turned on.] In the presence of high plasma levels of insulin, HSL is dephosphorylated and inactivated. Insulin also suppresses expression of ATGL. [Note: Fat droplets are coated by a protein (perilipin) that limits access of HSL. Phosphorylation of perilipin by PKA allows translocation and binding of HSL to the droplet.]
2. Fate of glycerol: The glycerol released during TAG degradation cannot be metabolized by adipocytes because they lack glycerol kinase. Rather, glycerol is transported through the blood to the liver, where it can be phosphorylated. The resulting glycerol 3-phosphate can be used to form TAG in the liver or can be converted to DHAP by reversal of the glycerol 3-phosphate dehydrogenase reaction illustrated in Figure 16.13. DHAP can participate in glycolysis or gluconeogenesis.
3. Fate of fatty acids: The free (unesterified) fatty acids move through the cell membrane of the adipocyte and bind to plasma albumin. They are transported to the tissues, enter cells, get activated to their CoA derivatives, and are oxidized for energy in mitochondria. Regardless of their levels, plasma FFAs cannot be used for fuel by red blood cells (RBCs), which have no mitochondria. Brain, too, does not use fatty acids for energy, but the reasons are less clear. [Note: Over 50% of the fatty acids released from adipose TAG are reesterified to glycerol 3-phosphate. WAT does not express glycerol kinase, and the phosphorylated glycerol is produced by glyceroneogenesis, an incomplete version of gluconeogenesis: pyruvate to OAA via pyruvate carboxylase and OAA to phosphoenolpyruvate (PEP) via phosphoenolpyruvate carboxykinase. The PEP is converted (by reactions common to glycolysis and gluconeogenesis) to DHAP, which is reduced to glycerol 3-phosphate. The process reduces plasma FFAs, molecules associated with insulin resistance in type 2 diabetes and obesity (see p. 343).]
Figure 16.15 Hormonal regulation of fat degradation in the adipocyte. [Note: Triacylglycerol is degraded to diacylglycerol by adipose triglyceride lipase.] cAMP = cyclic adenosine monophosphate; PPi = pyrophosphate; ADP = adenosine diphosphate; ![]() = phosphate.
= phosphate.
B. β-Oxidation of fatty acids
The major pathway for catabolism of fatty acids is a mitochondrial pathway called β-oxidation, in which two-carbon fragments are successively removed from the carboxyl end of the fatty acyl CoA, producing acetyl CoA, NADH, and flavin adenine dinucleotide (FADH2).
1. Transport of long-chain fatty acids into mitochondria: After a LCFA enters a cell, it is converted in the cytosol to its CoA derivative by long-chain fatty acyl CoA synthetase (thiokinase), an enzyme of the outer mitochondrial membrane. Because β-oxidation occurs in the mitochondrial matrix, the fatty acid must be transported across the inner mitochondrial membrane that is impermeable to CoA. Therefore, a specialized carrier transports the long-chain acyl group from the cytosol into the mitochondrial matrix. This carrier is carnitine, and this rate-limiting transport process is called the “carnitine shuttle” (Figure 16.16).
Figure 16.16 Carnitine shuttle. The net effect is that a long-chain fatty acyl coenzyme A (CoA) is transported from the outside to the inside of mitochondria. AMP = adenosine monophosphate; PPi = pyrophosphate.
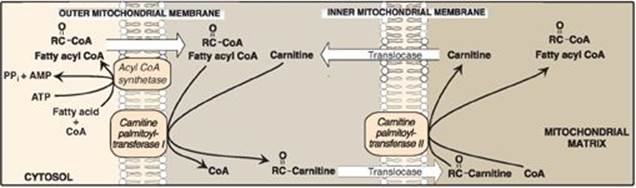
a. Steps in translocation: First, the acyl group is transferred from CoA to carnitine by carnitine palmitoyltransferase I (CPT-I), an enzyme of the outer mitochondrial membrane. [Note: CPT-I is also known as CAT-I for carnitine acyltransferase I.] This reaction forms an acylcarnitine and regenerates free CoA. Second, the acylcarnitine is transported into the mitochondrial matrix in exchange for free carnitine by carnitine–acylcarnitine translocase. Carnitine palmitoyltransferase II (CPT-II, or CAT-II), an enzyme of the inner mitochondrial membrane, catalyzes the transfer of the acyl group from carnitine to CoA in the mitochondrial matrix, thus regenerating free carnitine.
b. Inhibitor of the carnitine shuttle: Malonyl CoA inhibits CPT-I, thus preventing the entry of long-chain acyl groups into the mitochondrial matrix. Therefore, when fatty acid synthesis is occurring in the cytosol (as indicated by the presence of malonyl CoA), the newly made palmitate cannot be transferred into mitochondria and degraded. [Note: Muscle, although it does not synthesize fatty acids, contains the mitochondrial isoform of ACC (ACC2 ), allowing muscle to regulate β-oxidation.] Fatty acid oxidation is also regulated by the acetyl CoA to CoA ratio: as the ratio increases, the CoA-requiring thiolase reaction decreases (Figure 16.17).
c. Sources of carnitine: Carnitine can be obtained from the diet, where it is found primarily in meat products. Carnitine can also be synthesized from the amino acids lysine and methionine by an enzymatic pathway found in the liver and kidney but not in skeletal or heart muscle. Therefore, these latter tissues are totally dependent on uptake of carnitine provided by endogenous synthesis or the diet and distributed by the blood. [Note: Skeletal muscle contains about 97% of all carnitine in the body.]
d. Carnitine deficiencies: Such deficiencies result in a decreased ability of tissues to use LCFAs as a fuel. Primary carnitine deficiency is caused by defects in a membrane transporter that prevent uptake of carnitine by cardiac and skeletal muscle and kidney. Treatment includes carnitine supplementation. Secondary carnitine deficiency occurs primarily as a result of defects in fatty acid oxidation leading to the accumulation of acylcarnitines that are excreted in the urine, decreasing carnitine availability. Acquired secondary carnitine deficiency can be seen, for example, in patients with liver disease (decreased carnitine synthesis) or those taking the antiseizure drug valproic acid (decreased renal reabsorption). [Note: Defects in mitochondrial oxidation can also be caused by deficiencies in CPT-I and CPT-II. CPT-I deficiency affects the liver, where an inability to use LCFAs for fuel greatly impairs that tissue’s ability to synthesize glucose (an endergonic process) during a fast. This can lead to severe hypoglycemia, coma, and death. CPT-II deficiency can affect the liver and cardiac and skeletal muscle. The most common (and least severe) form affects skeletal muscle. It presents as muscle weakness with myoglobinemia following prolonged exercise. Treatment includes avoidance of fasting and adopting a diet high in carbohydrates and low in fat but supplemented with medium-chain TAGs.]
2. Entry of short- and medium-chain fatty acids into the mitochondria: Fatty acids shorter than 12 carbons can cross the inner mitochondrial membrane without the aid of carnitine or the CPT system. Once inside the mitochondria, they are activated to their CoA derivatives by matrix enzymes, and are oxidized. [Note: Medium-chain fatty acids are plentiful in human milk. Because their oxidation is not dependent on CPT-I, it is not subject to inhibition by malonyl CoA.]
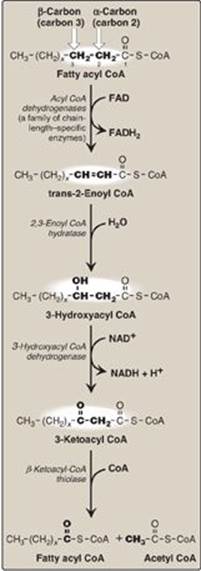
3. Reactions of β-oxidation: The first cycle of β-oxidation is shown in Figure 16.17. It consists of a sequence of four reactions involving the β-carbon (carbon 3) that results in shortening the fatty acid chain by two carbons at the carboxylate end. The steps include an oxidation that produces FADH2, a hydration step, a second oxidation that produces NADH, and a thiolytic cleavage that releases a molecule of acetyl CoA. Each step is catalyzed by enzymes with chain-length specificity. These four steps are repeated for saturated fatty acids of even-numbered carbon chains (n/2) - 1 times (where n is the number of carbons), each cycle producing one acetyl CoA plus one NADH and one FADH2. The acetyl CoA can be oxidized or used in hepatic ketogenesis (see below). The reduced coenzymes are oxidized by the electron transport chain. The final thiolytic cleavage produces two acetyl groups. [Note: Acetyl CoA is a positive allosteric effector of pyruvate carboxylase (see p. 119), thus linking fatty acid oxidation and gluconeogenesis.]
Figure 16.17 Enzymes involved in the β-oxidation of fatty acyl coenzyme A (CoA). [Note: 2,3-Enoyl CoA hydratase requires a trans double bond between carbon 2 and carbon 3.] FAD(H2) = flavin adenine dinucleotide; NAD(H) = nicotinamide adenine dinucleotide.
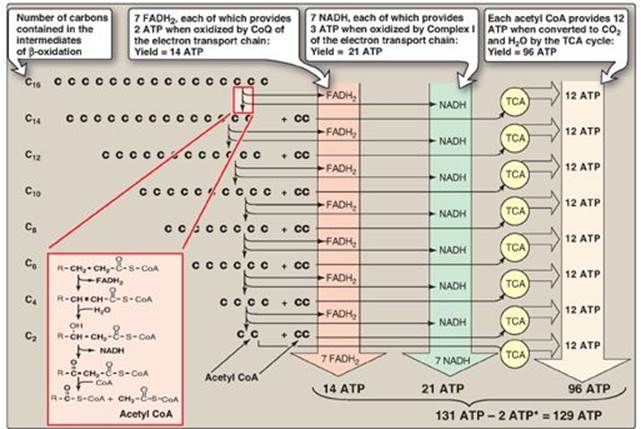
4. Energy yield from fatty acid oxidation: The energy yield from the β-oxidation pathway is high. For example, the oxidation of a molecule of palmitoyl CoA to CO2 and H2O produces 8 acetyl CoA, 7 NADH, and 7 FADH2, from which 131 ATP can be generated. However, activation of the fatty acid requires 2 ATP. Therefore, the net yield from palmitate is 129 ATP (Figure 16.18). A comparison of the processes of synthesis and degradation of long-chain saturated fatty acids with an even number of carbon atoms is provided in Figure 16.19.
5. Medium-chain fatty acyl CoA dehydrogenase deficiency: In mitochondria, there are four fatty acyl CoA dehydrogenase species, each with distinct but overlapping specificity for either short-, medium-, long-, or VLCFAs. Medium-chain fatty acyl CoA dehydrogenase (MCAD) deficiency, an autosomal-recessive disorder, is one of the most common inborn errors of metabolism and the most common inborn error of fatty acid oxidation, being found in 1:14,000 births worldwide, with a higher incidence in Caucasians of Northern European descent. It results in decreased ability to oxidize fatty acids with six to ten carbons (which accumulate and can be measured in urine), severe hypoglycemia (because the tissues must increase their reliance on glucose), and hypoketonemia (because of decreased production of acetyl CoA). See below. Treatment includes avoidance of fasting. MCADdeficiency has been identified as the cause of some cases originally reported as sudden infant death syndrome or Reye syndrome.
Figure 16.18 Summary of the energy yield from the oxidation of palmitoyl coenzyme A (CoA) (16 carbons). *Activation of palmitate to palmitoyl CoA requires the equivalent of 2 ATP (ATP → AMP + PPi). FADH2 = flavin adenine dinucleotide; NADH = nicotinamide adenine dinucleotide; TCA = tricarboxylic acid; CoQ = coenzyme Q.
6. Oxidation of fatty acids with an odd number of carbons: This process proceeds by the same reaction steps as that of fatty acids with an even number of carbons, until the final three carbons are reached. This compound, propionyl CoA, is metabolized by a three-step pathway (Figure 16.20). [Note: Propionyl CoA is also produced during the metabolism of certain amino acids (see Figure 20.10).]
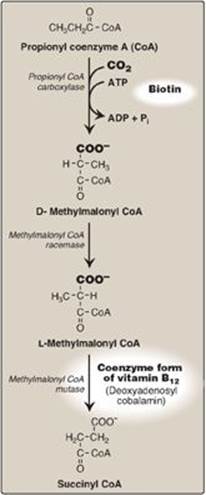
a. Synthesis of D-methylmalonyl coenzyme A: First, propionyl CoA is carboxylated, forming D-methylmalonyl coenzyme A. The enzyme propionyl CoA carboxylase has an absolute requirement for the coenzyme biotin, as do ACC and most other carboxylases.
Figure 16.19 Comparison of the synthesis and degradation of long-chain, even-numbered, saturated fatty acids. NADPH = nicotinamide adenine dinucleotide phosphate; NAD = nicotinamide adenine dinucleotide; FAD = flavin adenine dinucleotide; CoA = coenzyme A.
b. Formation of L-methylmalonyl coenzyme A: Next, the D-isomer is converted to the L-form by the enzyme, methylmalonyl CoA racemase.
c. Synthesis of succinyl coenzyme A: Finally, the carbons of L-methylmalonyl CoA are rearranged, forming succinyl CoA, which can enter the tricarboxylic acid (TCA) cycle (see p. 109). [Note: This is the only example of a glucogenic precursor generated from fatty acid oxidation.] The enzyme methylmalonyl CoA mutase requires a coenzyme form of vitamin B12 (deoxyadenosylcobalamin). The mutase reaction is one of only two reactions in the body that require vitamin B12 (see p. 375). [Note: In patients with vitamin B12 deficiency, both propionate and methylmalonate are excreted in the urine. Two types of heritable methylmalonic acidemia and aciduria have been described: one in which the mutase is missing or deficient (or has reduced affinity for the coenzyme), and one in which the patient is unable to convert vitamin B12 into its coenzyme form. Either type results in metabolic acidosis and neurologic manifestations.]
7. Oxidation of unsaturated fatty acids: The oxidation of unsaturated fatty acids provides less energy than that of saturated fatty acids because unsaturated fatty acids are less highly reduced, and, therefore, fewer reducing equivalents can be produced from these structures. Oxidation of monounsaturated fatty acids, such as 18:1(9) (oleic acid), requires one additional enzyme, 3,2-enoyl CoA isomerase, which converts the 3-cis derivative obtained after three rounds of β-oxidation to the 2-trans derivative required as a substrate by the enoyl CoA hydratase. Oxidation of polyunsaturated fatty acids, such as 18:2(9,12) (linoleic acid), requires an NADPH-dependent 2,4-dienoyl CoA reductase in addition to the isomerase.
8. β-Oxidation in the peroxisome: VLCFAs of more than 22 carbons undergo a preliminary β-oxidation in peroxisomes, because peroxisomes are the primary site of the synthetase that activates fatty acids of this length. The shortened fatty acid (linked to carnitine) diffuses to a mitochondrion for further oxidation. In contrast to mitochondrial β-oxidation, the initial dehydrogenation in peroxisomes is catalyzed by a FAD-containing acyl CoA oxidase. The FADH2 produced is oxidized by molecular oxygen, which is reduced to H2O2. Therefore, no ATP is generated by this step. The H2O2 is reduced to H2O by catalase (see p. 148). [Note: Genetic defects in the ability either to target matrix proteins to peroxisomes (resulting in Zellweger syndrome, a peroxisomal biogenesis disorder) or to transport VLCFAs across the peroxisomal membrane (resulting in X-linked adrenoleukodystrophy), lead to accumulation of VLCFAs in the blood and tissues.]
Figure 16.20 Metabolism of propionyl CoA. ADP = adenosine diphosphate; Pi = inorganic phosphate.
C. Peroxisomal α-oxidation of fatty acids
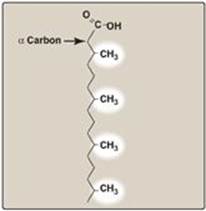
Branched-chain phytanic acid: This product of chlorophyll metabolism is not a substrate for acyl CoA dehydrogenase because of the methyl group on its β-carbon (Figure 16.21). Instead, it is hydroxylated at the α-carbon by phytanoyl CoA α-hydroxylase (PhyH), carbon 1 is released as CO2, and the product, 19-carbon pristanal, is oxidized to pristanic acid, which is activated to its CoA derivative and undergoes β-oxidation. Refsum disease is a rare, autosomal-recessive disorder caused by a deficiency of peroxisomal PhyH. This results in the accumulation of phytanic acid in the plasma and tissues. The symptoms are primarily neurologic, and the treatment involves dietary restriction to halt disease progression. [Note: ω-Oxidation (at the methyl terminus) also is known and generates dicarboxylic acids. Normally a minor pathway of the ER, its upregulation is seen with conditions such as MCADdeficiency that limit fatty acid β-oxidation.]
Figure 16.21 Phytanic acid, a branched-chain fatty acid.
V. KETONE BODIES: AN ALTERNATE FUEL FOR CELLS
Liver mitochondria have the capacity to convert acetyl CoA derived from fatty acid oxidation into ketone bodies. The compounds categorized as ketone bodies are acetoacetate, 3-hydroxybutyrate (also called β-hydroxybutyrate), and acetone (a nonmetabolized side product, Figure 16.22). [Note: The two functional ketone bodies are actually organic acids.] Acetoacetate and 3-hydroxybutyrate are transported in the blood to the peripheral tissues. There they can be reconverted to acetyl CoA, which can be oxidized by the TCA cycle. Ketone bodies are important sources of energy for the peripheral tissues because 1) they are soluble in aqueous solution and, therefore, do not need to be incorporated into lipoproteins or carried by albumin as do the other lipids; 2) they are produced in the liver during periods when the amount of acetyl CoA present exceeds the oxidative capacity of the liver; and 3) they are used in proportion to their concentration in the blood by extrahepatic tissues, such as the skeletal and cardiac muscle, intestinal mucosa, and renal cortex. Even the brain can use ketone bodies to help meet its energy needs if the blood levels rise sufficiently. Thus, ketone bodies spare glucose, which is particularly important during prolonged periods of fasting (see p. 332). [Note: Disorders of fatty acid oxidation present with the general picture of hypoketosis (due to decreased availability of acetyl CoA) and hypoglycemia (due to increased reliance on glucose for energy.]
A. Synthesis of ketone bodies by the liver: ketogenesis
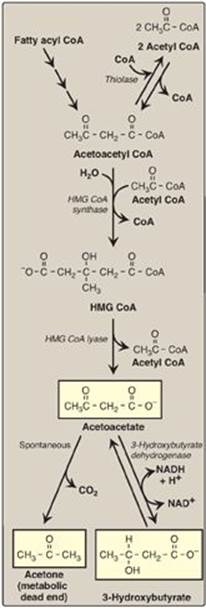
During a fast, the liver is flooded with fatty acids mobilized from adipose tissue. The resulting elevated hepatic acetyl CoA produced by fatty acid oxidation inhibits pyruvate dehydrogenase (see p. 111), and activates pyruvate carboxylase (see p. 119). The OAA produced is used by the liver for gluconeogenesis rather than for the TCA cycle. Therefore, acetyl CoA is channeled into ketone body synthesis. Additionally, fatty acid oxidation decreases the NAD+ to NADH ratio, and the rise in NADH shifts OAA to malate (see p. 113). This also pushes acetyl CoA into ketogenesis (Figure 16.24).] [Note: Acetyl CoA for ketogenesis is also generated by the catabolism of ketogenic amino acids (see p. 262).]
1. Synthesis of 3-hydroxy-3-methylglutaryl coenzyme A: The first step, formation of acetoacetyl CoA, occurs by reversal of the thiolase reaction of fatty acid oxidation (see Figure 16.17). Mitochondrial 3-hydroxy-3-methylglutaryl (HMG) CoA synthase combines a third molecule of acetyl CoA with acetoacetyl CoA to produce HMG CoA. HMG CoA synthase is the rate-limiting step in the synthesis of ketone bodies and is present in significant quantities only in the liver. [Note: HMG CoA is also an intermediate in cytosolic cholesterol synthesis (see p. 220). The two pathways are separated by location in, and conditions of, the cell.]
2. Synthesis of the ketone bodies: HMG CoA is cleaved by HMG CoA lyase to produce acetoacetate and acetyl CoA, as shown in Figure 16.22. Acetoacetate can be reduced to form 3-hydroxybutyrate with NADH as the hydrogen donor. Acetoacetate can also spontaneously decarboxylate in the blood to form acetone, a volatile, biologically nonmetabolized compound that can be released in the breath. The equilibrium between acetoacetate and 3-hydroxybutyrate is determined by the NAD+/NADH ratio. Because this ratio is low during fatty acid oxidation, 3-hydroxybutyrate synthesis is favored. [Note: The generation of free CoA during ketogenesis allows fatty acid oxidation to continue.]
Figure 16.22 Synthesis of ketone bodies. [Note: The release of CoA in ketogenesis supports continued fatty acid oxidation.] CoA = coenzyme A; HMG = hydroxymethylglutarate; NAD(H) = nicotinamide adenine dinucleotide.
B. Use of ketone bodies by the peripheral tissues: ketolysis
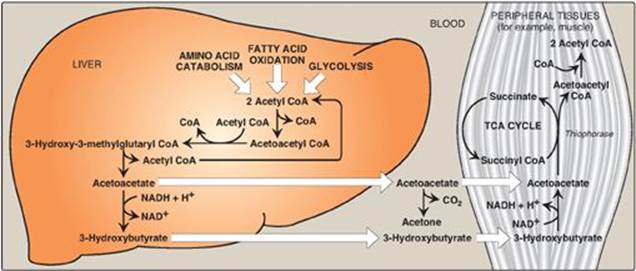
Although the liver constantly synthesizes low levels of ketone bodies, their production becomes much more significant during fasting when ketone bodies are needed to provide energy to the peripheral tissues. 3-Hydroxybutyrate is oxidized to acetoacetate by 3-hydroxybutyrate dehydrogenase, producing NADH (Figure 16.23). Acetoacetate is then provided with a CoA molecule taken from succinyl CoA by succinyl CoA:acetoacetate CoA transferase(thiophorase). This reaction is reversible, but the product, acetoacetyl CoA, is actively removed by its conversion to two acetyl CoAs. This pulls the reaction forward. Extrahepatic tissues, including the brain but excluding cells lacking mitochondria (for example, RBCs), efficiently oxidize acetoacetate and 3-hydroxybutyrate in this manner. In contrast, although the liver actively produces ketone bodies, it lacks thiophorase and, therefore, is unable to use ketone bodies as fuel.
Figure 16.23 Ketone body synthesis in the liver and use in peripheral tissues. Liver and red blood cells cannot use ketone bodies. [Note: Thiophorase is also known as succinyl CoA:acetoacetate CoA transferase.] CoA = coenzyme A; NAD(H) = nicotinamide adenine dinucleotide; TCA = tricarboxylic acid.
C. Excessive production of ketone bodies in diabetes mellitus
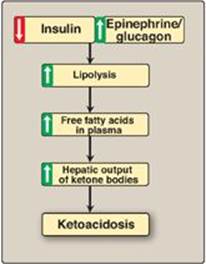
When the rate of formation of ketone bodies is greater than the rate of their use, their levels begin to rise in the blood (ketonemia) and, eventually, in the urine (ketonuria). This is seen most often in cases of uncontrolled type 1 diabetes mellitus. In diabetic individuals with severe ketosis, urinary excretion of the ketone bodies may be as high as 5,000 mg/24 hr, and the blood concentration may reach 90 mg/dl (versus less than 3 mg/dl in normal individuals). A frequent symptom of diabetic ketoacidosis (DKA) is a fruity odor on the breath, which results from increased production of acetone. An elevation of the ketone body concentration in the blood results in acidemia. [Note: The carboxyl group of a ketone body has a pKa of about 4. Therefore, each ketone body loses a proton (H+) as it circulates in the blood, which lowers the pH. Also, in DKA, urinary loss of glucose and ketone bodies results in dehydration. Therefore, the increased number of H+ circulating in a decreased volume of plasma can cause severe acidosis (ketoacidosis).] Ketoacidosis may also be seen in cases of prolonged fasting (see p. 330) and excessive ethanol consumption (see p. 318).
Figure 16.24 Mechanism of diabetic ketoacidosis seen in type 1 diabetes.
VI. CHAPTER SUMMARY
Generally a linear hydrocarbon chain with a terminal carboxyl group, a fatty acid can be saturated or unsaturated. Two fatty acids are dietary essentials: linoleic and α-linolenic acids. Fatty acids are synthesized in the cytosol of liver following a meal containing excess carbohydrate and protein. Carbons used to synthesize fatty acids are provided by acetyl coenzyme A (CoA), energy by ATP, and reducing equivalents by nicotinamide adenine dinucleotide phosphate ([NADPH]; Figure 16.25) provided by the pentose phosphate pathway and malic enzyme. Citrate carries two-carbon acetyl units from the mitochondrial matrix to the cytosol. The regulated step in fatty acid synthesis is catalyzed by biotin-requiring acetyl CoA carboxylase (ACC). Citrate allosterically activates ACC and long-chain fatty acyl CoAs inhibit it. ACC can also be activated by insulin and inactivated by adenosine monophosphate–activated protein kinase (AMPK) in response to epinephrine, glucagon, or a rise in AMP. The remaining steps in fatty acid synthesis are catalyzed by the multifunctional enzyme, fatty acid synthase, which produces palmitoyl CoA by adding two-carbon units from malonyl CoA to a series of acyl acceptors. Fatty acids can be elongated and desaturated in the endoplasmic reticulum (ER). When fatty acids are required for energy, adipocyte hormone-sensitive lipase (activated by epinephrine, and inhibited by insulin), along with other lipases, degrades stored triacylglycerol (TAG). The fatty acid products are carried by serum albumin to the liver and peripheral tissues, where oxidation of the fatty acids provides energy. The glycerol backbone of the degraded TAG is carried by the blood to the liver, where it serves as an important gluconeogenic precursor. Fatty acid degradation (β-oxidation) occurs in mitochondria. The carnitine shuttle is required to transport long-chain fatty acids from the cytosol to the mitochondrial matrix. A translocase and the enzymes carnitine palmitoyltransferases (CPT) I and II are required. CPT-I is inhibited by malonyl CoA, thereby preventing simultaneous synthesis and degradation of fatty acids. In the mitochondria, fatty acids are oxidized, producing acetyl CoA, nicotinamide adenine dinucleotide (NADH), and flavin adenine dinucleotide (FADH2). The first step in the β-oxidation pathway is catalyzed by one of four acyl CoA dehydrogenases, each with chain-length specificity. Medium-chain fatty acyl CoA dehydrogenase (MCAD) deficiency causes a decrease in fatty acid oxidation (process stops once a medium chain fatty acid is produced), resulting in hypoketonemia and severe hypoglycemia. Oxidation of fatty acids with an odd number of carbons proceeds two carbons at a time (producing acetyl CoA) until three-carbon propionyl CoA remains. This compound is carboxylated to methylmalonyl CoA (by biotin-requiring propionyl CoA carboxylase), which is then converted to succinyl CoA (a gluconeogenic precursor) by vitamin B2-requiring methylmalonyl CoA mutase. A genetic error in the mutase or vitamin B12 deficiency causes methylmalonic acidemia and aciduria. β-Oxidation of very-long-chain fatty acids and α-oxidation of branched-chain fatty acids occur in the peroxisome. ω-Oxidation, a minor pathway, occurs in the ER. Liver mitochondria can convert acetyl CoA derived from fatty acid oxidation into the ketone bodies acetoacetate and 3-hydroxybutyrate. Peripheral tissues possessing mitochondria can oxidize 3-hydroxybutyrate to acetoacetate, which can be reconverted to acetyl CoA, thereby producing energy for the cell. Unlike fatty acids, ketone bodies are utilized by the brain and, therefore, are important fuels during a fast. Because the liver lacks the ability to degrade ketone bodies, it synthesizes them specifically for the peripheral tissues. Ketoacidosis occurs when the rate of ketone body formation is greater than the rate of use, as is seen in cases of uncontrolled type 1 diabetes mellitus.
Study Questions
Choose the ONE correct answer.
16.1 When oleic acid, 18:1(9), is desaturated at carbon 6 and then elongated, what is the product?
A. 19:2(7,9)
B. 20:2 (n-6)
C. 20:2(6,9)
D. 20:2(8,11)
Correct answer = D. Fatty acids are elongated in the endoplasmic reticulum by adding two carbons at a time to the carboxylate end (carbon 1) of the molecule. This pushes the double bonds at carbon 6 and carbon 9 further away from carbon 1. 20:2(8,11) is an n-9 (ω-9) fatty acid.
Figure 16.25 Key concept map for fatty acid and triacylglycerol metabolism. AMPK = adenosine monophosphate-activated protein kinase; PKA = protein kinase A; CoA = coenzyme A; NADP(H) = nicotinamide adenine dinucleotide phosphate; FAD(H2) = flavin adenine dinucleotide; NAD(H) = nicotinamide adenine dinucleotide; TCA = tricarboxylic acid; VLDL = very-low-density lipoprotein.
16.2 A 4-month-old child is being evaluated for fasting hypoglycemia. Laboratory tests at admission reveal low levels of ketone bodies, free carnitine, and acylcarnitines in the blood. Free fatty acid levels in the blood were elevated. Deficiency of which of the following would best explain these findings?
A. Adipose triglyceride lipase
B. Carnitine transporter
C. Carnitine palmitoyltransferase I
D. Long-chain fatty acid dehydrogenase
Correct answer = B. A defect in the carnitine transporter (primary carnitine deficiency) would result in low levels of carnitine in the blood (as a result of increased urinary loss) and low levels in the tissues. In the liver, this decreases fatty acid oxidation and ketogenesis. Consequently, blood levels of free fatty acids rise. Deficiencies of adipose triglyceride lipase would decrease fatty acid availability. Deficiency of carnitine palmitoyltransferase I would result in elevated blood carnitine. Defects in any of the enzymes of β-oxidation would result in secondary carnitine deficiency, with a rise in acylcarnitines.
16.3 A teenager, concerned about his weight, attempts to maintain a fat-free diet for a period of several weeks. If his ability to synthesize various lipids were examined, he would be found to be most deficient in his ability to synthesize:
A. cholesterol.
B. glycolipids.
C. phospholipids.
D. prostaglandins.
E. triacylglycerol.
Correct answer = D. Prostaglandins are synthesized from arachidonic acid. Arachidonic acid is synthesized from linoleic acid, an essential fatty acid obtained by humans from dietary lipids. The teenager would be able to synthesize all other compounds but, presumably, in somewhat decreased amounts.
16.4 A 6-month-old boy was hospitalized following a seizure. History revealed that for several days prior, his appetite was decreased due to a “stomach virus.” At admission, his blood glucose was 24 mg/dl (age-referenced normal is 60–100). His urine was negative for ketone bodies and positive for a variety of dicarboxylic acids. Blood carnitine levels were normal. A tentative diagnosis of medium-chain fatty acyl coenzyme A dehydrogenase (MCAD) deficiency is made. In patients with MCAD deficiency, the fasting hypoglycemia is a consequence of:
A. decreased acetyl coenzyme A production.
B. decreased ability to convert acetyl coenzyme A to glucose.
C. increased conversion of acetyl coenzyme A to acetoacetate.
D. increased production of ATP and nicotinamide adenine dinucleotide.
Correct answer = A. Impaired oxidation of fatty acids less than 12 carbons in length results in decreased production of acetyl coenzyme (CoA), the allosteric activator of pyruvate carboxylase, a gluconeogenic enzyme, and, thus, glucose levels fall. Acetyl CoA can never be used for the net synthesis of glucose. Acetoacetate is a ketone body, and with medium-chain fatty acyl CoA dehydrogenase deficiency, ketogenesis is decreased as a result of decreased production of the substrate, acetyl CoA. Impaired fatty acid oxidation means that less ATP and nicotinamide adenine dinucleotide are made, and both are needed for gluconeogenesis.
16.5 Explain why with Zellweger syndrome both very-long-chain fatty acids (VLCFAs) and phytanic acid accumulate, whereas with X-linked adrenoleukodystrophy, only VLCFAs accumulate.
Zellweger syndrome is caused by an inability to target matrix proteins to the peroxisome. Therefore, all peroxisomal activities are affected because functional peroxisomes are not able to be formed. In X-linked adrenoleukodystrophy, the defect is an inability to transport very-long-chain fatty acids into the peroxisome, but other peroxisomal functions, such as α-oxidation, are normal.