CHEMISTRY THE CENTRAL SCIENCE
18 CHEMISTRY OF THE ENVIRONMENT
18.4 HUMAN ACTIVITIES AND EARTH'S WATER
All life on Earth depends on the availability of suitable water. Some organisms can thrive under temperature, pH, and ionic conditions where other organisms would die. Many human activities rely on waste disposal via Earth's waters, even today, and this practice can be detrimental to aquatic organisms.
Dissolved Oxygen and Water Quality
The amount of O2 dissolved in water is an important indicator of water quality. Water fully saturated with air at 1 atm and 20 °C contains about 9 ppm of O2. Oxygen is necessary for fish and most other aquatic life. Cold-water fish require water containing at least 5 ppm of dissolved oxygen for survival. Aerobic bacteria consume dissolved oxygen to oxidize organic materials for energy. The organic material the bacteria are able to oxidize is said to be biodegradable.

![]() FIGURE 18.18 Eutrophication. This rapid accumulation of dead and decaying plant matter in a body of water uses up the water's oxygen supply, making the water unsuitable for aquatic animals.
FIGURE 18.18 Eutrophication. This rapid accumulation of dead and decaying plant matter in a body of water uses up the water's oxygen supply, making the water unsuitable for aquatic animals.
Excessive quantities of biodegradable organic materials in water are detrimental because they remove the oxygen necessary to sustain normal animal life. Typical sources of these biodegradable materials, which are called oxygen-demanding wastes, include sewage, industrial wastes from food-processing plants and paper mills, and liquid waste from meatpacking plants.
![]() GO FIGURE
GO FIGURE
What feature of this process is responsible for its being called reverse osmosis?
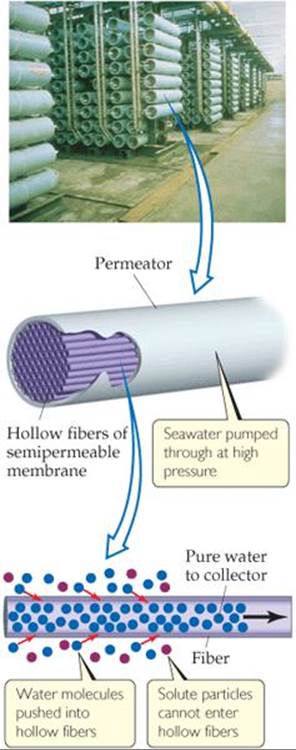
![]() FIGURE 18.19 Reverse osmosis.
FIGURE 18.19 Reverse osmosis.
In the presence of oxygen, the carbon, hydrogen, nitrogen, sulfur, and phosphorus in biodegradable material end up mainly as CO2, HCO3–, H2O, NO3–, SO42–, and phosphates. The formation of these oxidation products sometimes reduces the amount of dissolved oxygen to the point where aerobic bacteria can no longer survive. Anaerobic bacteria then take over the decomposition process, forming CH4, NH3, H2S, PH3, and other products, several of which contribute to the offensive odors of some polluted waters.
Plant nutrients, particularly nitrogen and phosphorus, contribute to water pollution by stimulating excessive growth of aquatic plants. The most visible results of excessive plant growth are floating algae and murky water. What is more significant, however, is that as plant growth becomes excessive, the amount of dead and decaying plant matter increases rapidly, a process called eutrophication (![]() FIGURE 18.18). The processes by which plants decay consumes O2, and without sufficient oxygen, the water cannot sustain animal life.
FIGURE 18.18). The processes by which plants decay consumes O2, and without sufficient oxygen, the water cannot sustain animal life.
The most significant sources of nitrogen and phosphorus compounds in water are domestic sewage (phosphate-containing detergents and nitrogen-containing body wastes), runoff from agricultural land (fertilizers contain both nitrogen and phosphorus), and runoff from livestock areas (animal wastes contain nitrogen).
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
If a test on a sample of polluted water shows a considerable decrease in dissolved oxygen over a five-day period, what can we conclude about the nature of the pollutants present?
Water Purification: Desalination
Because of its high salt content, seawater is unfit for human consumption and for most of the uses to which we put water. In the United States the salt content of municipal water supplies is restricted by health codes to no more than about 0.05% by mass. This amount is much lower than the 3.5% dissolved salts present in seawater and the 0.5% or so present in brackish water found underground in some regions. The removal of salts from seawater or brackish water to make the water usable is called desalination.
Water can be separated from dissolved salts by distillation because water is a volatile substance and the salts are nonvolatile. ![]() (Section 13.5: “A Closer Look: Ideal Solutions with Two or More Volatile Components”) The principle of distillation is simple enough, but carrying out the process on a large scale presents many problems. As water is distilled from seawater, for example, the salts become more and more concentrated and eventually precipitate out. Distillation is also an energy-intensive process.
(Section 13.5: “A Closer Look: Ideal Solutions with Two or More Volatile Components”) The principle of distillation is simple enough, but carrying out the process on a large scale presents many problems. As water is distilled from seawater, for example, the salts become more and more concentrated and eventually precipitate out. Distillation is also an energy-intensive process.
Seawater can also be desalinated using reverse osmosis. Recall that osmosis is the net movement of solvent molecules, but not solute molecules, through a semipermeable membrane. ![]() (Section 13.5) In osmosis, the solvent passes from the more dilute solution into the more concentrated one. However, if sufficient external pressure is applied, osmosis can be stopped and, at still higher pressures, reversed. When reverse osmosis occurs, solvent passes from the more concentrated into the more dilute solution. In a modern reverse-osmosis facility, hollow fibers are used as the semipermeable membrane (
(Section 13.5) In osmosis, the solvent passes from the more dilute solution into the more concentrated one. However, if sufficient external pressure is applied, osmosis can be stopped and, at still higher pressures, reversed. When reverse osmosis occurs, solvent passes from the more concentrated into the more dilute solution. In a modern reverse-osmosis facility, hollow fibers are used as the semipermeable membrane (![]() FIGURE 18.19). Water is introduced under pressure into the fibers, and desalinated water is recovered.
FIGURE 18.19). Water is introduced under pressure into the fibers, and desalinated water is recovered.
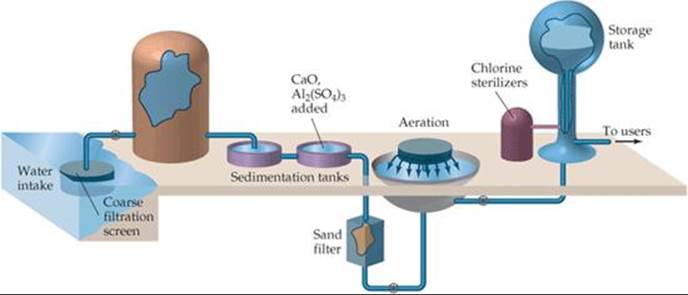
![]() FIGURE 18.20 Common steps in treating water for a public water system.
FIGURE 18.20 Common steps in treating water for a public water system.
The world's largest desalination plant, in Jubail, Saudi Arabia, provides 50% of that country's drinking water by using reverse osmosis to desalinate seawater from the Persian Gulf. Such plants are becoming increasingly common in the United States. The largest, near Tampa Bay, Florida, has been operating since 2007 and produces 35 million gallons of drinking water a day by reverse osmosis. Small-scale, manually operated reverse-osmosis desalinators are used in camping, traveling, and at sea.
Water Purification: Municipal Treatment
The water needed for domestic, agricultural, and industrial use is taken either from lakes, rivers, and underground sources or from reservoirs. Much of the water that finds its way into municipal water systems is “used” water, meaning it has already passed through one or more sewage systems or industrial plants. Consequently, this water must be treated before it is distributed to our faucets.
Municipal water treatment usually involves five steps (![]() FIGURE 18.20). After coarse filtration through a screen, the water is allowed to stand in large sedimentation tanks where sand and other minute particles settle out. To aid in removing very small particles, the water may first be made slightly basic with CaO. Then Al2(SO4)3 is added and reacts with OH– ions to form a spongy, gelatinous precipitate of Al(OH)3 (Ksp = 1.3 × 10–33). This precipitate settles slowly, carrying suspended particles down with it, thereby removing nearly all finely divided matter and most bacteria. The water is then filtered through a sand bed. Following filtration, the water may be sprayed into the air (aeration) to hasten oxidation of dissolved organic substances.
FIGURE 18.20). After coarse filtration through a screen, the water is allowed to stand in large sedimentation tanks where sand and other minute particles settle out. To aid in removing very small particles, the water may first be made slightly basic with CaO. Then Al2(SO4)3 is added and reacts with OH– ions to form a spongy, gelatinous precipitate of Al(OH)3 (Ksp = 1.3 × 10–33). This precipitate settles slowly, carrying suspended particles down with it, thereby removing nearly all finely divided matter and most bacteria. The water is then filtered through a sand bed. Following filtration, the water may be sprayed into the air (aeration) to hasten oxidation of dissolved organic substances.
The final step normally involves treating the water with a chemical agent to ensure the destruction of bacteria. Ozone is more effective, but chlorine is less expensive. Liquefied Cl2 is dispensed from tanks through a metering device directly into the water supply. The amount used depends on the presence of other substances with which the chlorine might react and on the concentrations of bacteria and viruses to be removed. The sterilizing action of chlorine is probably due not to Cl2 itself but to hypochlorous acid, which forms when chlorine reacts with water:
![]()
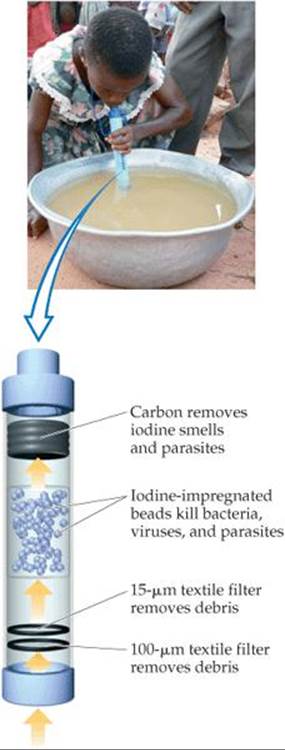
![]() FIGURE 18.21 A LifeStraw purifies water as it is drunk.
FIGURE 18.21 A LifeStraw purifies water as it is drunk.
As many as a billion people worldwide lack access to clean water. According to the United Nations, 95% of the world's cities still dump raw sewage into their water supplies. Thus, it should come as no surprise that 80% of all the health maladies in developing countries can be traced to waterborne diseases associated with unsanitary water.
One promising development is a device called the LifeStraw (![]() FIGURE 18.21). When a person sucks water through the straw, the water first encounters a textile filter with a mesh opening of 100 µm followed by a second textile filter with a mesh opening of 15 µm. These filters remove debris and even clusters of bacteria. The water next encounters a chamber of iodine-impregnated beads, where bacteria, viruses, and parasites are killed. Finally, the water passes through granulated active carbon, which removes the smell of iodine as well as the parasites that have not been taken by the filters or killed by the iodine.
FIGURE 18.21). When a person sucks water through the straw, the water first encounters a textile filter with a mesh opening of 100 µm followed by a second textile filter with a mesh opening of 15 µm. These filters remove debris and even clusters of bacteria. The water next encounters a chamber of iodine-impregnated beads, where bacteria, viruses, and parasites are killed. Finally, the water passes through granulated active carbon, which removes the smell of iodine as well as the parasites that have not been taken by the filters or killed by the iodine.
 A CLOSER LOOK
A CLOSER LOOK
WATER SOFTENING
Water containing a relatively high concentration of Ca2+, Mg2+, and other divalent cations is called hard water. Although the presence of these ions is generally not a health threat, they can make water unsuitable for some household and industrial uses. For example, these ions react with soap to form an insoluble soap scum, the stuff of bathtub rings.
In addition, mineral deposits may form when water containing these ions is heated. When water containing calcium ions and bicarbonate ions is heated, some carbon dioxide is driven off. As a result, the solution becomes less acidic and insoluble calcium carbonate forms:
![]()
The solid CaCO3 coats the surface of hot-water systems and teakettles, reducing heating efficiency (![]() FIGURE 18.22). These deposits, called scale, can be especially serious in boilers where water is heated under pressure in pipes running through a furnace.
FIGURE 18.22). These deposits, called scale, can be especially serious in boilers where water is heated under pressure in pipes running through a furnace.
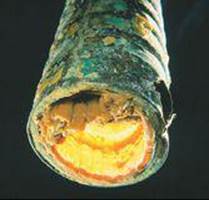
![]() FIGURE 18.22 Scale formation. The interior of this water pipe has been coated with CaCO3 and other insoluble salts deposited from hard water.
FIGURE 18.22 Scale formation. The interior of this water pipe has been coated with CaCO3 and other insoluble salts deposited from hard water.
Removal of the ions that cause hard water is called water softening. In the lime-soda process used for large-scale municipal water-softening operations, the water is treated with calcium hydroxide (prepared from lime) and, sodium carbonate (sometimes called soda ash) to precipitate Ca2+ as CaCO3 and Mg2+ as Mg(OH)2:
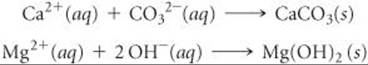
In ion exchange, a typical household method for softening water, hard water is passed through an ion-exchange resin made up of plastic beads with covalently bound anion groups such as —COO– or —SO3–. These negatively charged groups have Na+ ions to balance their charges. The Ca2+ and other cations in the hard water are attracted to the anionic groups and displace the lower-charged Na+ into the water. Thus, one type of ion is exchanged for another. To maintain charge balance, 2 Na+ enter the water for each Ca2+ removed. If we represent the resin with its anionic site as R —COO–, we can write the equation for the process as
![]()
Water softened in this way contains an increased concentration of Na+. Although Na+ does not form precipitates or cause other problems associated with hard-water cations, individuals concerned about their sodium intake, such as those who have high blood pressure (hypertension), should avoid drinking water softened in this way.
When all the available Na+ ions have been displaced from the ion-exchange resin, the resin is regenerated by flushing it with a concentrated solution of NaCl. The high concentration of Na+ forces the equilibrium in the preceding equation to shift to the left, causing the Na+ to displace the hard-water cations, which are flushed down the drain.
Water disinfection is one of the greatest public health innovations in human history. It has dramatically decreased the incidences of waterborne bacterial diseases such as cholera and typhus. However, this great benefit comes at a price.
In 1974 scientists in Europe and the United States discovered that chlorination of water produces a group of by-products previously undetected. These by-products are called trihalomethanes (THMs) because all have a single carbon atom and three halogen atoms: CHCl3, CHCl2Br, CHClBr2, and CHBr3. These and many other chlorine- and bromine-containing organic substances are produced by the reaction of dissolved chlorine with the organic materials present in nearly all natural waters, as well as with substances that are by-products of human activity. Recall that chlorine dissolves in water to form the oxidizing agent HClO:
![]()
The HClO in turn reacts with organic substances to form THMs. Bromine enters the reaction sequence through the reaction of HClO with dissolved bromide ion:
![]()
Then both HBrO(aq) and HClO(aq) can halogenate organic substances to form the THMs.
Some THMs and other halogenated organic substances are suspected carcinogens; others interfere with the body's endocrine system. As a result, the World Health Organization and the U.S. Environmental Protection Agency have placed concentration limits of 80 μg/L (80 ppb) on the total quantity of THMs in drinking water. The goal is to reduce the levels of THMs and other disinfection by-products in the drinking water supply while preserving the antibacterial effectiveness of the water treatment. In some cases, lowering the concentration of chlorine may provide adequate disinfection while reducing the concentrations of THMs formed. Alternative oxidizing agents, such as ozone or chlorine dioxide, produce less of the halogenated substances but have their own disadvantages. For example, each is capable of oxidizing dissolved bromide, as shown here for ozone:
![]()
![]()
Bromate ion, BrO3–, has been shown to cause cancer in animal tests. Bromate's cancer-causing potential has led Los Angeles, for example, to add 3 million black plastic balls to one of its drinking water reservoirs to prevent bromate from forming via photochemical processes (![]() FIGURE 18.23).
FIGURE 18.23).

![]() FIGURE 18.23 Preventing photochemical bromate reactions. The black plastic balls added to this Los Angeles drinking water reservoir keep sunlight from entering the water and initiating reactions that form harmful bromate ions.
FIGURE 18.23 Preventing photochemical bromate reactions. The black plastic balls added to this Los Angeles drinking water reservoir keep sunlight from entering the water and initiating reactions that form harmful bromate ions.
At present, there seem to be no completely satisfactory alternatives to chlorination or ozonation, and we are faced with a consideration of benefit versus risk. In this case, the risks of cancer from THMs and related substances in municipal water are very low relative to the risks of cholera, typhus, and gastrointestinal disorders from untreated water. When the water supply is cleaner to begin with, less disinfectant is needed and thus the risk of THMs is lowered. Once THMs form, their concentrations in the water supply can be reduced by aeration because the THMs are more volatile than water. Alternatively, they can be removed by adsorption onto activated charcoal or other adsorbents.