CHEMISTRY THE CENTRAL SCIENCE
20 ELECTRO-CHEMISTRY
20.3 VOLTAIC CELLS
The energy released in a spontaneous redox reaction can be used to perform electrical work. This task is accomplished through a voltaic (or galvanic) cell, a device in which the transfer of electrons takes place through an external pathway rather than directly between reactants present in the same reaction vessel.
One such spontaneous reaction occurs when a strip of zinc is placed in contact with a solution containing Cu2+. As the reaction proceeds, the blue color of Cu2+(aq) ions fades, and copper metal deposits on the zinc. At the same time, the zinc begins to dissolve. These transformations, shown in ![]() FIGURE 20.3, are summarized by the equation
FIGURE 20.3, are summarized by the equation
![]()
![]() FIGURE 20.4 shows a voltaic cell that uses the redox reaction given in Equation 20.7. Although the setup in Figure 20.4 is more complex than that in Figure 20.3, the reaction is the same in both cases. The significant difference is that in the voltaic cell the Zn metal and Cu2+(aq) are not in direct contact with each other. Instead, Zn metal is in contact with Zn2+(aq) in one compartment, and Cu metal is in contact with Cu2+(aq) in the other compartment. Consequently, Cu reduction can occur only by the flow of electrons through an external circuit, namely, a wire connecting the Zn and Cu strips. Electrons flowing through a wire and ions moving in solution both constitute an electrical current. This flow of electrical charge can be used to accomplish electrical work.
FIGURE 20.4 shows a voltaic cell that uses the redox reaction given in Equation 20.7. Although the setup in Figure 20.4 is more complex than that in Figure 20.3, the reaction is the same in both cases. The significant difference is that in the voltaic cell the Zn metal and Cu2+(aq) are not in direct contact with each other. Instead, Zn metal is in contact with Zn2+(aq) in one compartment, and Cu metal is in contact with Cu2+(aq) in the other compartment. Consequently, Cu reduction can occur only by the flow of electrons through an external circuit, namely, a wire connecting the Zn and Cu strips. Electrons flowing through a wire and ions moving in solution both constitute an electrical current. This flow of electrical charge can be used to accomplish electrical work.
![]() GO FIGURE
GO FIGURE
Which metal, Cu or Zn, is oxidized in this voltaic cell?

![]() FIGURE 20.4 A Cu-Zn voltaic cell based on the reaction in Equation 20.7.
FIGURE 20.4 A Cu-Zn voltaic cell based on the reaction in Equation 20.7.
The two solid metals connected by the external circuit are called electrodes. By definition, the electrode at which oxidation occurs is the anode and the electrode at which reduction occurs is the cathode.* The electrodes can be made of materials that participate in the reaction, as in the present example. Over the course of the reaction, the Zn electrode gradually disappears and the copper electrode gains mass. More typically, the electrodes are made of a conducting material, such as platinum or graphite, that does not gain or lose mass during the reaction but serves as a surface at which electrons are transferred.
Each compartment of a voltaic cell is called a half-cell. One half-cell is the site of the oxidation half-reaction, and the other is the site of the reduction half-reaction. In our present example, Zn is oxidized and Cu2+ is reduced:
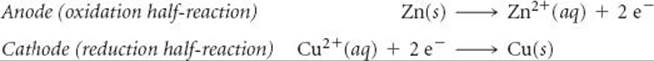
Electrons become available as zinc metal is oxidized at the anode. They flow through the external circuit to the cathode, where they are consumed as Cu2+(aq) is reduced. Because Zn(s) is oxidized in the cell, the zinc electrode loses mass, and the concentration of the Zn2+ solution increases as the cell operates. At the same time, the Cu electrode gains mass, and the Cu2+ solution becomes less concentrated as Cu2+ is reduced to Cu(s).
For a voltaic cell to work, the solutions in the two half-cells must remain electrically neutral. As Zn is oxidized in the anode half-cell, Zn2+ ions enter the solution, upsetting the initial Zn2+/SO42+ charge balance. To keep the solution electrically neutral, there-must be some means for Zn2+ cations to migrate out of the anode half-cell and for anions to migrate in. Similarly, the reduction of Cu2+ at the cathode removes these cations from the solution, leaving an excess of SO42– anions in that half-cell. To maintain electrical neutrality, some of these anions must migrate out of the cathode half-cell, and positive ions must migrate in. In fact, no measurable electron flow occurs between electrodes unless a means is provided for ions to migrate through the solution from one half-cell to the other, thereby completing the circuit.
In Figure 20.4, a porous glass disc separating the two half-cells allows ions to migrate and maintain the electrical neutrality of the solutions. In ![]() FIGURE 20.5, a salt bridgeserves this purpose. The salt bridge consists of a U-shaped tube containing an electrolyte solution, such as NaNO3(aq), whose ions will not react with other ions in the voltaic cell or with the electrodes. The electrolyte is often incorporated into a paste or gel so that the electrolyte solution does not pour out when the U-tube is inverted. As oxidation and reduction proceed at the electrodes, ions from the salt bridge migrate into the two half-cells—cations migrating to the cathode half-cell and anions migrating to the anode half-cell—to neutralize charge in the half-cell solutions. Whichever device is used to allow ions to migrate between half-cells, anions always migrate toward the anode and cations toward the cathode.
FIGURE 20.5, a salt bridgeserves this purpose. The salt bridge consists of a U-shaped tube containing an electrolyte solution, such as NaNO3(aq), whose ions will not react with other ions in the voltaic cell or with the electrodes. The electrolyte is often incorporated into a paste or gel so that the electrolyte solution does not pour out when the U-tube is inverted. As oxidation and reduction proceed at the electrodes, ions from the salt bridge migrate into the two half-cells—cations migrating to the cathode half-cell and anions migrating to the anode half-cell—to neutralize charge in the half-cell solutions. Whichever device is used to allow ions to migrate between half-cells, anions always migrate toward the anode and cations toward the cathode.
![]() GO FIGURE
GO FIGURE
How is electrical balance maintained in the left beaker as Zn2+ ions are formed at the anode?
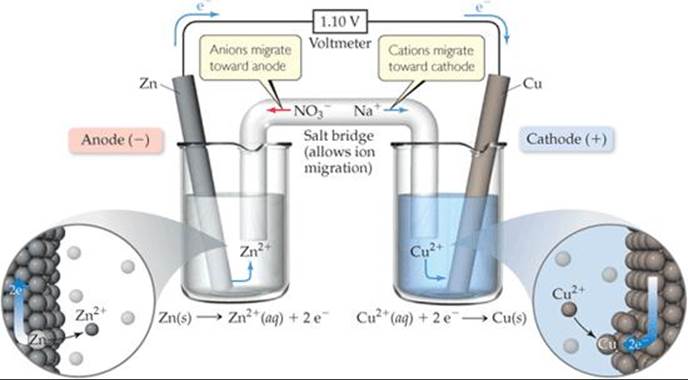
![]() FIGURE 20.5 A voltaic cell that uses a salt bridge to complete the electrical circuit.
FIGURE 20.5 A voltaic cell that uses a salt bridge to complete the electrical circuit.

![]() FIGURE 20.6 Summary of reactions occurring in a voltaic cell. The half-cells can be separated by either a porous glass disc (as in Figure 20.4) or by a salt bridge (as in Figure 20.5).
FIGURE 20.6 Summary of reactions occurring in a voltaic cell. The half-cells can be separated by either a porous glass disc (as in Figure 20.4) or by a salt bridge (as in Figure 20.5).
![]() FIGURE 20.6 summarizes the various relationships in a voltaic cell. Notice in particular that electrons flow from the anode through the external circuit to the cathode. Because of this directional flow, the anode in a voltaic cell is labeled with a negative sign and the cathode is labeled with a positive sign. We can envision the electrons as being attracted to the positive cathode from the negative anode through the external circuit.
FIGURE 20.6 summarizes the various relationships in a voltaic cell. Notice in particular that electrons flow from the anode through the external circuit to the cathode. Because of this directional flow, the anode in a voltaic cell is labeled with a negative sign and the cathode is labeled with a positive sign. We can envision the electrons as being attracted to the positive cathode from the negative anode through the external circuit.
SAMPLE EXERCISE 20.4 Describing a Voltaic Cell
The oxidation-reduction reaction
![]()
is spontaneous. A solution containing K2Cr2O7 and H2SO4 is poured into one beaker, and a solution of KI is poured into another. A salt bridge is used to join the beakers. A metallic conductor that will not react with either solution (such as platinum foil) is suspended in each solution, and the two conductors are connected with wires through a voltmeter or some other device to detect an electric current. The resultant voltaic cell generates an electric current. Indicate the reaction occurring at the anode, the reaction at the cathode, the direction of electron migration, the direction of ion migration, and the signs of the electrodes.
SOLUTION
Analyze We are given the equation for a spontaneous reaction that takes place in a voltaic cell and a description of how the cell is constructed. We are asked to write the half-reactions occurring at the anode and at the cathode, as well as the directions of electron and ion movements and the signs assigned to the electrodes.
Plan Our first step is to divide the chemical equation into half-reactions so that we can identify the oxidation and the reduction processes. We then use the definitions of anode and cathode and the other terminology summarized in Figure 20.6.
Solve In one half-reaction, Cr2O72–(aq) is converted into Cr3+(aq). Starting with these ions and then completing and balancing the half-reaction, we have
![]()
In the other half-reaction, I–(aq) is converted to I2(s):
![]()
Now we can use the summary in Figure 20.6 to help us describe the voltaic cell. The first half-reaction is the reduction process (electrons on the reactant side of the equation). By definition, the reduction process occurs at the cathode. The second half-reaction is the oxidation process (electrons on the product side of the equation), which occurs at the anode.
The I– ions are the source of electrons, and the Cr2O72– ions accept the electrons. Hence, the electrons flow through the external circuit from the electrode immersed in the KI solution (the anode) to the electrode immersed in the K2Cr2O7–H2SO4 solution (the cathode). The electrodes themselves do not react in any way; they merely provide a means of transferring electrons from or to the solutions. The cations move through the solutions toward the cathode, and the anions move toward the anode. The anode (from which the electrons move) is the negative electrode, and the cathode (toward which the electrons move) is the positive electrode.
PRACTICE EXERCISE
The two half-reactions in a voltaic cell are
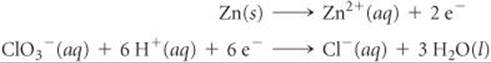
(a) Indicate which reaction occurs at the anode and which at the cathode. (b) Which electrode is consumed in the cell reaction? (c) Which electrode is positive?
Answers: (a) The first reaction occurs at the anode and the second reaction at the cathode. (b) The anode (Zn) is consumed in the cell reaction. (c) The cathode is positive.