CHEMISTRY THE CENTRAL SCIENCE
22 CHEMISTRY OF THE NONMETALS
22.7 NITROGEN
In 1772 the Scottish botanist Daniel Rutherford found that a mouse enclosed in a sealed jar quickly consumed the life-sustaining component of air (oxygen) and died. When the “fixed air” (CO2) in the container was removed, a “noxious air” remained that would not sustain combustion or life. We now know that gas as nitrogen.
Nitrogen constitutes 78% by volume of Earth's atmosphere, where it occurs as N2 molecules. Although nitrogen is a key element in living organisms, compounds of nitrogen are not abundant in Earth's crust. The major natural deposits of nitrogen compounds are those of KNO3(saltpeter) in India and NaNO3 (Chile saltpeter) in Chile and other desert regions of South America.
Properties of Nitrogen
Nitrogen is a colorless, odorless, tasteless gas composed of N2 molecules. Its melting point is –210 °C, and its normal boiling point is –196 °C.
The N2 molecule is very unreactive because of the strong triple bond between nitrogen atoms (the N ≡ N bond enthalpy is 941 kJ/mol, nearly twice that for the bond in O2; ![]() Table 8.4). When substances burn in air, they normally react with O2 but not with N2. When magnesium burns in air, however, it reacts with N2 to form magnesium nitride (Mg3N2):
Table 8.4). When substances burn in air, they normally react with O2 but not with N2. When magnesium burns in air, however, it reacts with N2 to form magnesium nitride (Mg3N2):
![]()
A similar reaction occurs with lithium, forming Li3N.
The nitride ion is a strong Brønsted–Lowry base and reacts with water to form ammonia:
![]()
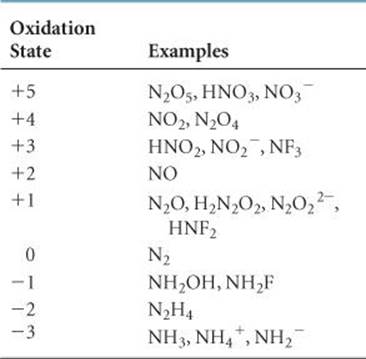
The electron configuration of the nitrogen atom is [He]2s 22p3. The element exhibits all formal oxidation states from +5 to –3 (![]() TABLE 22.6). The +5, 0, and –3 oxidation states are the most common and generally the most stable of these. Because nitrogen is more electronegative than all other elements except fluorine, oxygen, and chlorine, it exhibits positive oxidation states only in combination with these three elements.
TABLE 22.6). The +5, 0, and –3 oxidation states are the most common and generally the most stable of these. Because nitrogen is more electronegative than all other elements except fluorine, oxygen, and chlorine, it exhibits positive oxidation states only in combination with these three elements.
TABLE 22.6 • Oxidation States of Nitrogen
Production and Uses of Nitrogen
Elemental nitrogen is obtained in commercial quantities by fractional distillation of liquid air. About 4× 1010 kg (40 million tons) of N2 is produced annually in the United States.
Because of its low reactivity, large quantities of N2 are used as an inert gaseous blanket to exclude O2 in food processing, manufacture of chemicals, metal fabrication, and production of electronic devices. Liquid N2 is employed as a coolant to freeze foods rapidly.
The largest use of N2 is in the manufacture of nitrogen-containing fertilizers, which provide a source of fixed nitrogen. We have previously discussed nitrogen fixation in the “Chemistry and Life” box in Section 14.7 and in the “Chemistry Put to Work” box in Section 15.2. Our starting point in fixing nitrogen is the manufacture of ammonia via the Haber process. ![]() (Section 15.2) The ammonia can then be converted into a variety of useful, simple nitrogen-containing species (
(Section 15.2) The ammonia can then be converted into a variety of useful, simple nitrogen-containing species (![]() FIGURE 22.22).
FIGURE 22.22).
Hydrogen Compounds of Nitrogen
Ammonia is one of the most important compounds of nitrogen. It is a colorless, toxic gas that has a characteristic irritating odor. As noted in previous discussions, the NH3 molecule is basic (Kb = 1.8 × 10–5).![]() (Section 16.7)
(Section 16.7)
![]() GO FIGURE
GO FIGURE
In which of these species is the oxidation number of nitrogen +3?
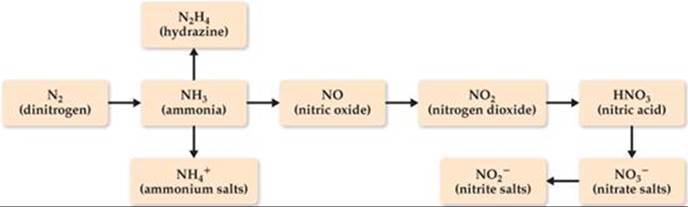
![]() FIGURE 22.22 Sequence of conversion of N2 into common nitrogen compounds.
FIGURE 22.22 Sequence of conversion of N2 into common nitrogen compounds.
In the laboratory, NH3 can be prepared by the action of NaOH on an ammonium salt. The NH4+ ion, which is the conjugate acid of NH3, transfers a proton to OH–. The resultant NH3 is volatile and is driven from the solution by mild heating:
![]()
Commercial production of NH3 is achieved by the Haber process:
![]()
About 1 × 1010 kg (10 million tons) of ammonia is produced annually in the United States. About 75% is used for fertilizer.
Hydrazine (N2H4) is another important hydride of nitrogen. The hydrazine molecule contains an N — N single bond (![]() FIGURE 22.23). Hydrazine is quite poisonous. It can be prepared by the reaction of ammonia with hypochlorite ion (OCl–) in aqueous solution:
FIGURE 22.23). Hydrazine is quite poisonous. It can be prepared by the reaction of ammonia with hypochlorite ion (OCl–) in aqueous solution:
![]()
![]() GO FIGURE
GO FIGURE
Is the N—N bond length in these molecules shorter or longer than the N—N bond length in N2?
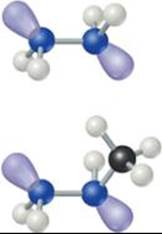
![]() FIGURE 22.23 Hydrazine (top, N2H4) and methylhydrazine (bottom, CH3NHNH2).
FIGURE 22.23 Hydrazine (top, N2H4) and methylhydrazine (bottom, CH3NHNH2).
The reaction involves several intermediates, including chloramine (NH2Cl). The poisonous NH2Cl bubbles out of solution when household ammonia and chlorine bleach (which contains OCl–) are mixed. This reaction is one reason for the frequently cited warning not to mix bleach and household ammonia.
Pure hydrazine is a strong and versatile reducing agent. The major use of hydrazine and compounds related to it, such as methylhydrazine (Figure 22.23), is as rocket fuel.
SAMPLE EXERCISE 22.7 Writing a Balanced Equation
Hydroxylamine (NH2OH) reduces copper(II) to the free metal in acid solutions. Write a balanced equation for the reaction, assuming that N2 is the oxidation product.
SOLUTION
Analyze We are asked to write a balanced oxidation-reduction equation in which NH2OH is converted to N2 and Cu2+ is converted to Cu.
Plan Because this is a redox reaction, the equation can be balanced by the method of half-reactions discussed in Section 20.2. Thus, we begin with two half-reactions, one involving the NH2OH and N2 and the other involving Cu2+ and Cu.
Solve The unbalanced and incomplete half-reactions are 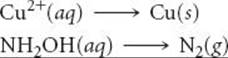
Balancing these equations as described in Section 20.2 gives 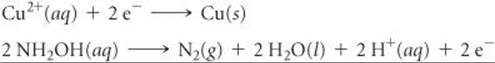
Adding these half-reactions gives the balanced equation: ![]()
PRACTICE EXERCISE
(a) In power plants, hydrazine is used to prevent corrosion of the metal parts of steam boilers by the O2 dissolved in the water. The hydrazine reacts with O2 in water to give N2 and H2O. Write a balanced equation for this reaction. (b) Methylhydrazine, N2H3CH3(l), is used with the oxidizer dinitrogen tetroxide, N2O4(l), to power the steering rockets of the Space Shuttle orbiter. The reaction of these two substances produces N2, CO2, and H2O. Write a balanced equation for this reaction.
Answer: ![]()
![]()
Oxides and Oxyacids of Nitrogen
Nitrogen forms three common oxides: N2O (nitrous oxide), NO (nitric oxide), and NO2 (nitrogen dioxide). It also forms two unstable oxides that we will not discuss, N2O3 (dinitrogen trioxide) and N2O5 (dinitrogen pentoxide).
Nitrous oxide (N2O) is also known as laughing gas because a person becomes giddy after inhaling a small amount. This colorless gas was the first substance used as a general anesthetic. It is used as the compressed gas propellant in several aerosols and foams, such as in whipped cream. It can be prepared in the laboratory by carefully heating ammonium nitrate to about 200 °C:
![]()
Nitric oxide (NO) is also a colorless gas but, unlike N2O, it is slightly toxic. It can be prepared in the laboratory by reduction of dilute nitric acid, using copper or iron as a reducing agent (![]() FIGURE 22.24):
FIGURE 22.24):
![]()
Nitric oxide is also produced by direct reaction of N2 and O2 at high temperatures. This reaction is a significant source of nitrogen oxide air pollutants. ![]() (Section 18.2) The direct combination of N2 and O2 is not used for commercial production of NO, however, because the yield is low, the equilibrium constant Kp at 2400 K being only 0.05.
(Section 18.2) The direct combination of N2 and O2 is not used for commercial production of NO, however, because the yield is low, the equilibrium constant Kp at 2400 K being only 0.05. ![]() (Section 15.7, “Chemistry Put to Work: Controlling Nitric Oxide Emissions”)
(Section 15.7, “Chemistry Put to Work: Controlling Nitric Oxide Emissions”)
The commercial route to NO (and hence to other oxygen-containing compounds of nitrogen) is via the catalytic oxidation of NH3:
![]()
This reaction is the first step in the Ostwald process, by which NH3 is converted commercially into nitric acid (HNO3).
![]() FIGURE 22.24 Copper metal reacts with nitric acid to produce blue Cu2+(aq) and NO(g).
FIGURE 22.24 Copper metal reacts with nitric acid to produce blue Cu2+(aq) and NO(g).
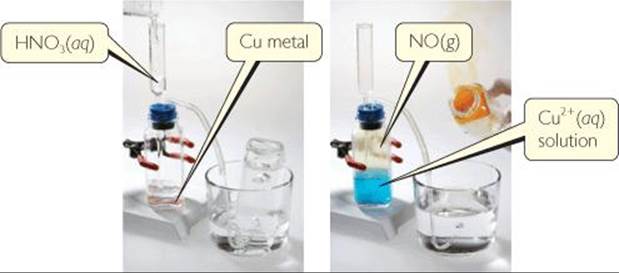
![]() FIGURE 22.25 Formation of NO2(g) as NO(g) combines with O2(g) in the air.
FIGURE 22.25 Formation of NO2(g) as NO(g) combines with O2(g) in the air.
When exposed to air, nitric oxide reacts readily with O2 (![]() FIGURE 22.25):
FIGURE 22.25):
![]()
When dissolved in water, NO2 forms nitric acid:
![]()
Nitrogen is both oxidized and reduced in this reaction, which means it disproportion-ates. The NO can be converted back into NO2 by exposure to air (Equation 22.45) and thereafter dissolved in water to prepare more HNO3.
NO is an important neurotransmitter in the human body. It causes the muscles that line blood vessels to relax, thus allowing an increased passage of blood (see the “Chemistry and Life” box on page 941).
Nitrogen dioxide (NO2) is a yellow-brown gas (Figure 22.25). Like NO, it is a major constituent of smog. ![]() (Section 18.2) It is poisonous and has a choking odor. As discussed in Section 15.1, NO2 and N2O4 exist in equilibrium:
(Section 18.2) It is poisonous and has a choking odor. As discussed in Section 15.1, NO2 and N2O4 exist in equilibrium:
![]()
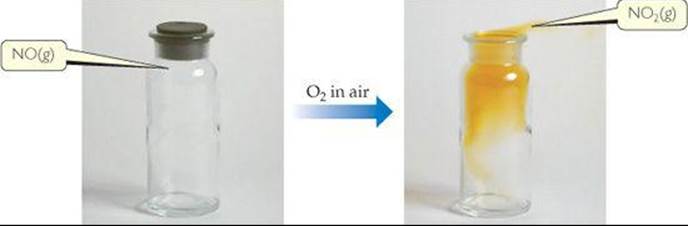
The two common oxyacids of nitrogen are nitric acid (HNO3) and nitrous acid (HNO2) (![]() FIGURE 22.26). Nitric acid is a strong acid. It is also a powerful oxidizing agent, as indicated by the standard reduction potential in the reaction
FIGURE 22.26). Nitric acid is a strong acid. It is also a powerful oxidizing agent, as indicated by the standard reduction potential in the reaction
![]()
![]() GO FIGURE
GO FIGURE
Which is the shortest NO bond in these two molecules?
![]() FIGURE 22.26 Structures of nitric acid (top) and nitrous acid (bottom).
FIGURE 22.26 Structures of nitric acid (top) and nitrous acid (bottom).
Concentrated nitric acid attacks and oxidizes most metals except Au, Pt, Rh, and Ir.
About 7 × 109 kg (8 million tons) of nitric acid is produced annually in the United States. Its largest use is in the manufacture of NH4NO3 for fertilizers. It is also used in the production of plastics, drugs, and explosives. Among the explosives made from nitric acid are nitroglycerin, trinitrotoluene (TNT), and nitrocellulose. The following reaction occurs when nitroglycerin explodes:
![]()
All the products of this reaction contain very strong bonds and are gases. As a result, the reaction is very exothermic, and the volume of the products is far larger than the volume occupied by the reactant. Thus, the expansion resulting from the heat generated by the reaction produces the explosion. ![]() (Section 8.8: “Chemistry Put to Work: Explosives and Alfred Nobel”)
(Section 8.8: “Chemistry Put to Work: Explosives and Alfred Nobel”)
Nitrous acid is considerably less stable than HNO3 and tends to disproportionate into NO and HNO3. It is normally made by action of a strong acid, such as H2SO4, on a cold solution of a nitrite salt, such as NaNO2. Nitrous acid is a weak acid (Ka = 4.5 × 10–4).
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
What are the oxidation numbers of the nitrogen atoms in
a. nitric acid;
b. nitrous acid?
 CHEMISTRY AND LIFE
CHEMISTRY AND LIFE
NITROGLYCERIN AND HEART DISEASE
During the 1870s an interesting observation was made in Alfred Nobel's dynamite factories. Workers who suffered from heart disease that caused chest pains when they exerted themselves found relief from the pains during the workweek. It quickly became apparent that nitroglycerin, present in the air of the factory, acted to enlarge blood vessels. Thus, this powerfully explosive chemical became a standard treatment for angina pectoris, the chest pains accompanying heart failure. It took more than 100 years to discover that nitroglycerin was converted in the vascular smooth muscle into NO, which is the chemical agent actually causing dilation of the blood vessels. In 1998 the Nobel Prize in Physiology or Medicine was awarded to Robert F. Furchgott, Louis J. Ignarro, and Ferid Murad for their discoveries of the detailed pathways by which NO acts in the cardiovascular system. It was a sensation that this simple, common air pollutant could exert important functions in mammals, including humans.
As useful as nitroglycerin is to this day in treating angina pectoris, it has a limitation in that prolonged administration results in development of tolerance, or desensitization, of the vascular muscle to further vasorelaxation by nitroglycerin. The bioactivation of nitroglycerin is the subject of active research in the hope that a means of circumventing desensitization can be found.