CHEMISTRY THE CENTRAL SCIENCE
23 TRANSITION METALS AND COORDINATION CHEMISTRY
EXERCISES
VISUALIZING CONCEPTS
23.1 This chart shows the variation in an important property of the metals from K through Ge. Is the property atomic radius, electronegativity, or first ionization energy? Explain your choice. [Section 23.1]

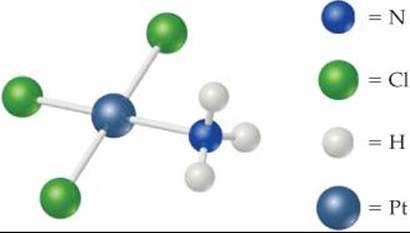
23.2 (a) Draw the structure for Pt(en)Cl2. (b) What is the coordination number for platinum in this complex, and what is the coordination geometry? (c) What is the oxidation state of the platinum? [Section 23.2]
23.3 Draw the Lewis structure for the ligand shown in the next column. (a) Which atoms can serve as donor atoms? Classify this ligand as monodentate, bidentate, or tridentate. (b) How many of these ligands are needed to fill the coordination sphere in an octahedral complex? [Section 23.2]
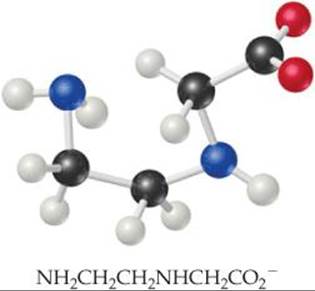
23.4 The complex ion shown here has a 1 – charge. Name the complex ion. [Section 23.4]
23.5 There are two geometric isomers of octahedral complexes of the type MA3X3, where M is a metal and A and X are mon-odentate ligands. Of the complexes shown here, which are identical to (1) and which are the geometric isomers of (1)? [Section 23.4]
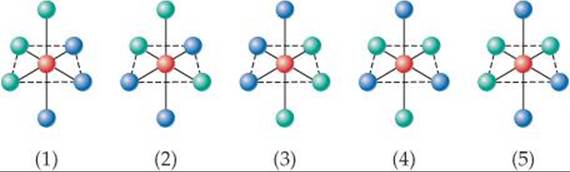
23.6 Which of the complexes shown here are chiral? Explain. [Section 23.4]
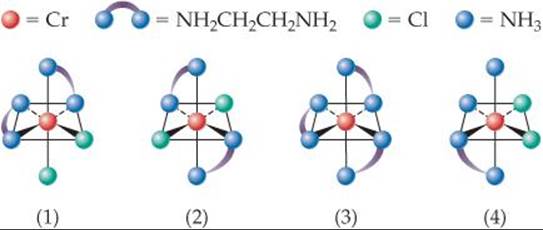
23.7 The solutions shown here each have an absorption spectrum with a single absorption peak like that shown in Figure 23.26. What color does each solution absorb most strongly? [Section 23.5]

23.8 Which of these crystal-field splitting diagrams represents: (a) a weak-field octahedral complex of Fe3+, (b) a strong-field octahedral complex of Fe3+, (c) a tetrahedral complex of Fe3+, (d) a tetrahedral complex of Ni2+? (The diagrams do not indicate the relative magnitudes of Δ.) [Section 23.6]
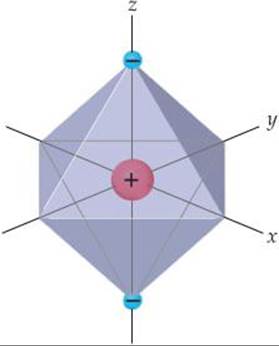
23.9 In the linear crystal field shown here, the negative charges are on the z-axis. Using Figure 23.28 as a guide, predict which d orbital has lobes closest to the charges. Which two have lobes farthest from the charges? Predict the crystal-field splitting of the d orbitals in linear complexes. [Section 23.6]
23.10 Two Fe(II) complexes are both low spin but have different li-gands. A solution of one is green and a solution of the other is red. Which solution is likely to contain the complex that has the stronger-field ligand? [Section 23.6]
THE TRANSITION METALS (section 23.1)
23.11 Explain the lanthanide contraction, and describe how it affects the properties of the transition-metal elements.
23.12 Sketch a plot of atomic radius versus number of valence d electrons for the period 5 transition metals, and explain the trend.
23.13 The +2 oxidation state is common for almost all the transition metals. Suggest an explanation.
23.14 No compounds are known in which scandium is in the +2 oxidation state. Suggest an explanation.
23.15 Write out the ground-state electron configurations of (a) Ti3+, (b) Ru2+, (c) Au3+, (d) Mn4+.
23.16 How many electrons are in the valence d orbitals in these transition-metal ions? (a) Co3+, (b) Cu+, (c) Cd2+, (d) Os3+.
23.17 Explain the difference between a diamagnetic substance and a paramagnetic substance.
23.18 Distinguish among a ferromagnetic substance, an antiferromagnetic substance, and a ferrimagnetic substance.
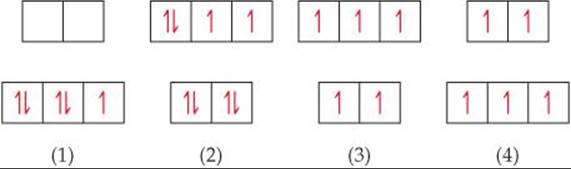
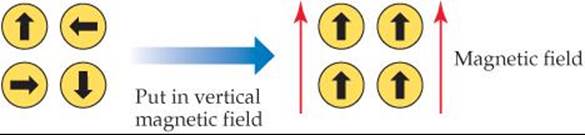
23.19 What kind of magnetism is exhibited by this diagram:
23.20 The most important oxides of iron are magnetite, Fe3O4, and hematite, Fe2O3. (a) What are the oxidation states of iron in these compounds? (b) One of these iron oxides is ferrimag-netic, and the other is antiferromagnetic. Which iron oxide is likely to show which type of magnetism? Explain.
TRANSITION-METAL COMPLEXES (section 23.2)
23.21 (a) What is the difference between Werner's concepts of primary valence and secondary valence? What terms do we now use for these concepts? (b) Why can the NH3 molecule serve as a ligand but the BH3 molecule cannot?
23.22 (a) What is the meaning of the term coordination number as it applies to metal complexes? (b) Give an example of a ligand that is neutral and one that is negatively charged. (c) Would you expect ligands that are positively charged to be common? Explain. (d) What type of chemical bonding is characteristic of coordination compounds? Illustrate with the compound Co(NH3)6Cl3. (e) What are the most common coordination numbers for metal complexes?
23.23 A complex is written as NiBr2 • 6 NH3. (a) What is the oxidation state of the Ni atom in this complex? (b) What is the likely coordination number for the complex? (c) If the complex is treated with excess AgNO3(aq), how many moles of AgBr will precipitate per mole of complex?
23.24 A certain complex of metal M is formulated as MCl3 • 3 H2O. The coordination number of the complex is not known but is expected to be 4 or 6. (a) Would conductivity measurements provide information about the coordination number? (b) In using conductivity measurements to test which ligands are bound to the metal ion, what assumption is made about the rate at which ligands enter or leave the coordination sphere of the metal? (c) Suppose you experimentally determine that this complex exists in aqueous solution as a single species. Suggest a likely coordination number and the number and type of each ligand.
23.25 Indicate the coordination number of the metal and the oxida tion number of the metal as well as the number and type of each donor atom of the ligands for each of the following complexes:
(a) Na2[CdCl4]
(b) K2[MoOCl4]
(c) [Co(NH3)4Cl2]Cl
(d) [Ni(CN)5]3–
(e) K3[V(C2O4)3]
(f) [Zn(en)2]Br2
23.26 Indicate the coordination number of the metal and the oxidation number of the metal as well as the number and type of each donor atom of the ligands for each of the following complexes:
(a) K3[Co(CN)6]
(b) Na2[CdBr4]
(c) [Pt(en)3](ClO4)4
(d) [Co(en)2(C2O4)]+
(e) NH4[Cr(NH3)2(NCS)4]
(f) [Cu(bipy)2I]I
COMMON LIGANDS IN COORDINATION CHEMISTRY (section 23.3)
23.27 (a) What is the difference between a monodentate ligand and a bidentate ligand? (b) How many bidentate ligands are necessary to fill the coordination sphere of a six-coordinate complex? (c) You are told that a certain molecule can serve as a tridentate ligand. Based on this statement, what do you know about the molecule?
23.28 For each of the following polydentate ligands, determine (i) the maximum number of coordination sites that the ligand can occupy on a single metal ion and (ii) the number and type of donor atoms in the ligand: (a) ethylenediamine (en), (b) bipyridine (bipy), (c) the oxalate anion (C2O42–), (d) the 2–ion of the porphine molecule (Figure 23.13); (e) [EDTA]4–.
23.29 Polydentate ligands can vary in the number of coordination positions they occupy. In each of the following, identify the polydentate ligand present and indicate the probable number of coordination positions it occupies:
(a) [Co(NH3)4(o-phen)]Cl3
(b) [Cr(C2O4)(H2O)4]Br
(c) [Cr(EDTA)(H2O)]–
(d) [Zn(en)2](ClO4)2
23.30 Indicate the likely coordination number of the metal in each of the following complexes:
(a) [Rh(bipy)3](NO3)3
(b) Na3[Co(C2O4)2Cl2]
(c) [Cr(o-phen)3](CH3COO)3
(d) Na2[Co(EDTA)Br]
23.31 (a) What is meant by the term chelate effect? (b) What thermo-dynamic factor is generally responsible for the chelate effect? (c) Why are polydentate ligands often called sequestering agents?
23.32 Pyridine (C5H5N), abbreviated py, is the molecule
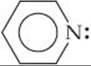
(a) Why is pyridine referred to as a monodentate ligand? (b) For the equilibrium reaction
![]()
what would you predict for the magnitude of the equilibrium constant? Explain your answer.
23.33 Is the following ligand a chelating one? Explain.
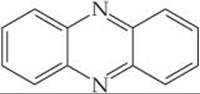
23.34 What is the geometry about the metal center in this complex? Would you expect this complex to have counterions? Explain.
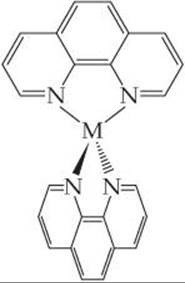
NOMENCLATURE AND ISOMERISM IN COORDINATION CHEMISTRY (section 23.4)
23.35 Write the formula for each of the following compounds, being sure to use brackets to indicate the coordination sphere:
(a) hexaamminechromium(III) nitrate
(b) tetraamminecarbonatocobalt(III) sulfate
(c) dichlorobis(ethylenediamine)platinum(IV) bromide
(d) potassium diaquatetrabromovanadate(III)
(e) bis(ethylenediamine)zinc(II) tetraiodomercurate(II)
23.36 Write the formula for each of the following compounds, being sure to use brackets to indicate the coordination sphere:
(a) tetraaquadibromomanganese(III) perchlorate
(b) bis(bipyridyl)cadmium(II) chloride
(c) potassium tetrabromo(ortho-phenanthroline)-cobaltate (III)
(d) cesium diamminetetracyanochromate(III)
(e) tris(ethylenediammine)rhodium(III) tris(oxalato)-cobaltate(III)
23.37 Write the names of the following compounds, using the standard nomenclature rules for coordination complexes:
(a) [Rh(NH3)4Cl2]Cl
(b) K2[TiCl6]
(c) MoOCl4
(d) [Pt(H2O)4(C2O4)]Br2
23.38 Write names for the following coordination compounds:
(a) [Cd(en)Cl2]
(b) K4[Mn(CN)6]
(c) [Cr(NH3)5CO3]Cl
(d) [Ir(NH3)4(H2O)2](NO3)3
23.39 By writing formulas or drawing structures related to any one of these three complexes,
[Co(NH3)4Br2]Cl
[Pd(NH3)2(ONO)2]
cis-[V(en)2Cl2]+,
illustrate (a) geometric isomerism, (b) linkage isomerism, (c) optical isomerism, (d) coordination-sphere isomerism.
23.40 (a) Draw the two linkage isomers of [Co(NH3)5SCN]2+.
(b) Draw the two geometric isomers of [Co(NH3)3Cl3]2+.
(c) Two compounds with the formula Co(NH3)5ClBr can be prepared. Use structural formulas to show how they differ. What kind of isomerism does this illustrate?
23.41 A four-coordinate complex MA2B2 is prepared and found to have two different isomers. Is it possible to determine from this information whether the complex is square planar or tetrahedral? If so, which is it?
23.42 Consider an octahedral complex MA3B3. How many geometric isomers are expected for this compound? Will any of the isomers be optically active? If so, which ones?
23.43 Sketch all the possible stereoisomers of (a) tetrahedral [Cd(H2O)2Cl2], (b) square-planar [IrCl2(PH3)2]–, (c) octahedral [Fe(o-phen)2Cl2]+.
23.44 Sketch all the possible stereoisomers of (a) [Rh(bipy)(o-phen)2]3+, (b) [Co(NH3)3(bipy)Br]2+, (c) square-planar [Pd(en)(CN)2].
COLOR AND MAGNETISM IN COORDINATION CHEMISTRY; CRYSTAL-FIELD THEORY (sections 23.5 and 23.6)
23.45 (a) Can we see light that is 300 nm in wavelength? 500 nm in wavelength? (b) What is meant by the term complementary color? (c) What is the significance of complementary colors in understanding the colors of metal complexes? (d) If a complex absorbs light at 610 nm, what is the energy of this absorption in kJ/mol?
23.46 (a) A complex absorbs light in the range of 200–300 nm. Do you expect it to have visible color? (b) A solution of a compound appears green. Does this observation necessarily mean that all colors of visible light other than green are absorbed by the solution? Explain. (c) What information is usually presented in a visible absorption spectrum of a compound? (d) What energy is associated with an absorption at 440 nm in kJ/mol?
23.47 Is it possible for a low-spin octahedral Fe(II) complex to be paramagnetic? Explain.
23.48 If a transition-metal complex has an even number of valence d electrons, does it necessarily mean that the complex is diamagnetic? Explain.
23.49 In crystal-field theory, ligands are modeled as if they are point negative charges. What is the basis of this assumption, and how does it relate to the nature of metal–ligand bonds?
23.50 Explain why the dxy, dxz, and dyz orbitals lie lower in energy than the dz2 and dx2–y2 orbitals in the presence of an octahedral arrangement of ligands about the central metal ion.
23.51 (a) Sketch a diagram that shows the definition of the crystal-field splitting energy (Δ) for an octahedral crystal field. (b) What is the relationship between the magnitude of Δ and the energy of the d-d transition for a d1 complex? (c) Calculate Δ in kJ/mol if a d1 complex has an absorption maximum at 545 nm.
23.52 As shown in Figure 23.26, the d-d transition of [Ti(H2O)6]3+ produces an absorption maximum at a wavelength of about 500 nm. (a) What is the magnitude of Δ for [Ti(H2O)6]3+ in kJ/mol? (b) What is the spectrochemical series? How would the magnitude of Δ change if the H2O ligands in [Ti(H2O)6]3+ were replaced with NH3 ligands?
23.53 Explain why many cyano complexes of divalent transition-metal ions are yellow, whereas many aqua complexes of these ions are blue or green.
23.54 The [Ni(H2O)6]2+ ion has an absorption maximum at about 725 nm, whereas the [Ni(NH3)6]2+ ion absorbs at about 570 nm. Predict the color of a solution of each ion. (b) The [Ni(en)3]2+ ion absorption maximum occurs at about 545 nm, and that of the [Ni(bipy)3]2+ ion occurs at about 520 nm. From these data, indicate the relative strengths of the ligand fields created by the four ligands involved.
23.55 Give the number of (valence) d electrons associated with the central metal ion in each of the following complexes: (a) K3[TiCl6], (b) Na3[Co(NO2)6], (c) [Ru(en)3]Br3, (d) [Mo(EDTA)]ClO4, (e) K3[ReCl6].
23.56 Give the number of (valence) d electrons associated with the central metal ion in each of the following complexes: (a) K3[Fe(CN)6], (b) [Mn(H2O)6](NO3)2, (c) Na[Ag(CN)2], (d) [Cr(NH3)4Br2]ClO4, (e) [Sr(EDTA)]2–.
23.57 A classmate says, “A weak-field ligand usually means the complex is high spin.” Is your classmate correct? Explain.
23.58 A classmate says, “A strong-field ligand means that the ligand binds strongly to the metal ion.” Is your classmate correct? Explain.
23.59 For each of the following metals, write the electronic configuration of the atom and its 2+ ion: (a) Mn, (b) Ru, (c) Rh. Draw the crystal-field energy-level diagram for the d orbitals of an octahedral complex, and show the placement of the d electrons for each 2+ ion, assuming a strong-field complex. How many unpaired electrons are there in each case?
23.60 For each of the following metals, write the electronic configuration of the atom and its 3+ ion: (a) Ru, (b) Mo, (c) Co. Draw the crystal-field energy-level diagram for the d orbitals of an octahedral complex, and show the placement of the d electrons for each 3+ ion, assuming a weak-field complex. How many unpaired electrons are there in each case?
23.61 Draw the crystal-field energy-level diagrams and show the placement of d electrons for each of the following: (a) [Cr(H2O)6]2+ (four unpaired electrons), (b) [Mn(H2O)6]2+ (high spin), (c) [Ru(NH3)5H2O]2+ (low spin), (d) [IrCl6]2– (low spin), (e) [Cr(en)3]3+, (f) [NiF6]4–.
23.62 Draw the crystal-field energy-level diagrams and show the placement of electrons for the following complexes: (a) [VCl6]3–, (b) [FeF6]3– (a high-spin complex), (c) [Ru(bipy)3]3+ (a low-spin complex), (d) [NiCl4]2– (tetrahedral), (e) [PtBr6]2–, (f) [Ti(en)3]2+.
23.63 The complex [Mn(NH3)6]2+ contains five unpaired electrons. Sketch the energy-level diagram for the d orbitals, and indicate the placement of electrons for this complex ion. Is the ion a high-spin or a low-spin complex?
23.64 The ion [Fe(CN)6]3– has one unpaired electron, whereas [Fe(NCS)6]3– has five unpaired electrons. From these results, what can you conclude about whether each complex is high spin or low spin? What can you say about the placement of NCS– in the spectrochemical series?
ADDITIONAL EXERCISES
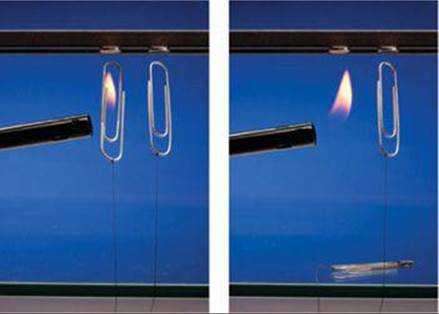
23.65 The Curie temperature is the temperature at which a ferromagnetic solid switches from ferromagnetic to paramagnetic, and for nickel, the Curie temperature is 354 °C. Knowing this, you tie a string to two paper clips made of nickel and hold the paper clips near a permanent magnet. The magnet attracts the paper clips, as shown in the photograph on the left. Now you heat one of the paper clips with a cigarette lighter, and the clip drops (right photograph). Explain what happened.
23.66 Explain why the transition metals in periods 5 and 6 have nearly identical radii in each group.
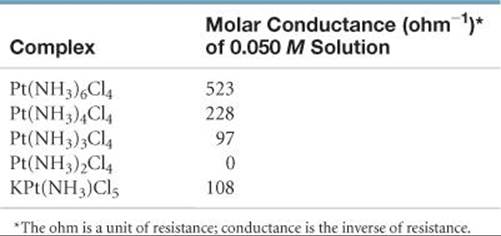
23.67 Based on the molar conductance values listed here for the series of platinum(IV) complexes, write the formula for each complex so as to show which ligands are in the coordination sphere of the metal. By way of example, the molar conductances of 0.050 M NaCl and BaCl2 are 107 ohm–1 and 197 ohm–1, respectively.
23.68 (a) A compound with formula RuCl3 • 5 H2O is dissolved in water, forming a solution that is approximately the same color as the solid. Immediately after forming the solution, the addition of excess AgNO3(aq) forms 2 mol of solid AgCl per mole of complex. Write the formula for the compound, showing which ligands are likely to be present in the coordination sphere. (b) After a solution of RuCl3 • 5 H2O has stood for about a year, addition of AgNO3(aq) precipitates 3 mol of AgCl per mole of complex. What has happened in the ensuing time?
23.69 Sketch the structure of the complex in each of the following compounds and give the full compound name:
(a) cis-[Co(NH3)4(H2O)2](NO3)2
(b) Na2[Ru(H2O)Cl5]
(c) trans-NH4[Co(C2O4)2(H2O)2]
(d) cis-[Ru(en)2Cl2]
23.70 (a) Which complex ions in Exercise 23.69 have a mirror plane? (b) Will any of the complexes be optically active? Explain.
23.71 The molecule dimethylphosphinoethane [(CH3)2PCH2-CH2P(CH3)2, which is abbreviated dmpe] is used as a ligand for some complexes that serve as catalysts. A complex that contains this ligand is Mo(CO)4(dmpe). (a) Draw the Lewis structure for dmpe, and compare it with ethylenediammine as a coordinating ligand. (b) What is the oxidation state of Mo in Na2[Mo(CN)2(CO)2(dmpe)]? (c) Sketch the structure of the [Mo(CN)2(CO)2(dmpe)]2– ion, including all the possible isomers.
23.72 Although the cis configuration is known for [Pt(en)Cl2], no trans form is known. (a) Explain why the trans compound is not possible. (b) Would NH2CH2CH2CH2CH2NH2 be more likely than en (NH2CH2CH2NH2) to form the trans compound? Explain.
23.73 The acetylacetone ion forms very stable complexes with many metallic ions. It acts as a bidentate ligand, coordinating to the metal at two adjacent positions. Suppose that one of the CH3 groups of the ligand is replaced by a CF3 group, as shown here,
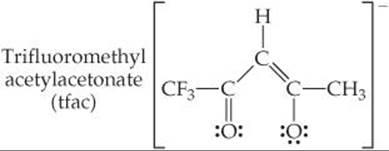
Sketch all possible isomers for the complex with three tfac li-gands on cobalt(III). (You can use the symbol ![]() to represent the ligand.)
to represent the ligand.)
23.74 Give brief statements about the relevance of the following complexes in living systems: (a) hemoglobin, (b) chlorophylls, (c) siderophores.
23.75 Write balanced chemical equations to represent the following observations. (In some instances the complex involved has been discussed previously in the text.) (a) Solid silver chloride dissolves in an excess of aqueous ammonia. (b) The green complex [Cr(en)2Cl2]Cl, on treatment with water over a long time, converts to a brown-orange complex. Reaction of AgNO3 with a solution of the product precipitates 3 mol of AgCl per mole of Cr present. (Write two chemical equations.) (c) When an NaOH solution is added to a solution of Zn(NO3)2, a precipitate forms. Addition of excess NaOH solution causes the precipitate to dissolve. (Write two chemical equations.) (d) A pink solution of Co(NO3)2 turns deep blue on addition of concentrated hydrochloric acid.
23.76 Some metal complexes have a coordination number of 5. One such complex is Fe(CO)5, which adopts a trigonal bipyramidal geometry (see Figure 9.8). (a) Write the name for Fe(CO)5, using the nomenclature rules for coordination compounds. (b) What is the oxidation state of Fe in this compound? (c) Suppose one of the CO ligands is replaced with a CN– lig and, forming [Fe(CO)4(CN)]-. How many geometric isomers would you predict this complex could have?
23.77 Which of the following objects is chiral: (a) a left shoe, (b) a slice of bread, (c) a wood screw, (d) a molecular model of Zn(en)Cl2, (e) a typical golf club?
23.78 The complexes [V(H2O)6]3+ and [VF6]3– are both known. (a) Draw the d-orbital energy-level diagram for V(III) octahedral complexes. (b) What gives rise to the colors of these complexes? (c) Which of the two complexes would you expect to absorb light of higher energy? Explain.
[23.79] One of the more famous species in coordination chemistry is the Creutz-Taube complex,
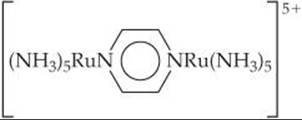
It is named for the two scientists who discovered it and initially studied its properties. The central ligand is pyrazine, a planar six-membered ring with nitrogens at opposite sides. (a) How can you account for the fact that the complex, which has only neutral ligands, has an odd overall charge? (b) The metal is in a low-spin configuration in both cases. Assuming octahedral coordination, draw the d-orbital energy-level dia gram for each metal. (c) In many experiments the two metal ions appear to be in exactly equivalent states. Can you think of a reason that this might appear to be so, recognizing that elec trons move very rapidly compared to nuclei?
23.80 Solutions of [Co(NH3)6]2+, [Co(H2O)6]2+ (both octahedral), and [CoCl4]2– (tetrahedral) are colored. One is pink, one is blue, and one is yellow. Based on the spectrochemical series and remembering that the energy splitting in tetrahe-dral complexes is normally much less than that in octahedral ones, assign a color to each complex.
23.81 Oxyhemoglobin, with an O2 bound to iron, is a low-spin Fe(II) complex; deoxyhemoglobin, without the O2 molecule, is a high-spin complex. (a) Assuming that the coordination environment about the metal is octahedral, how many unpaired electrons are centered on the metal ion in each case? (b) What ligand is coordinated to the iron in place of O2 in de-oxyhemoglobin? (c) Explain in a general way why the two forms of hemoglobin have different colors (hemoglobin is red, whereas deoxyhemoglobin has a bluish cast). (d) A 15-minute exposure to air containing 400 ppm of CO causes about 10% of the hemoglobin in the blood to be converted into the car bon monoxide complex, called carboxyhemoglobin. What does this suggest about the relative equilibrium constants for binding of carbon monoxide and O2 to hemoglobin?(e) CO is a strong-field ligand. What color might you expect carboxy-hemoglobin to be?
[23.82] Consider the tetrahedral anions VO43– (orthovanadate ion), CrO42–(chromate ion), and MnO4– (permanganate ion). (a) These anions are isoelectronic. What does this statement mean? (b) Would you expect these anions to exhibit d-d transitions? Explain. (c) As mentioned in “A Closer Look” on charge-transfer color, the violet color of MnO4– is due to a ligand-to-metal charge transfer (LMCT) transition. What is meant by this term? (d) The LMCT transition in MnO4– occurs at a wavelength of 565 nm. The CrO42– ion is yellow. Is the wavelength of the LMCT transition for chromate larger or smaller than that for MnO4–? Explain. (e) The VO43– ion is colorless. Do you expect the light absorbed by the LMCT to fall in the UV or the IR region of the electromagnetic spectrum? Explain your reasoning.
[23.83] Given the colors observed for VO43– (orthovanadate ion), CrO42– (chromate ion), and MnO4– (permanganate ion) (see Exercise 23.82), what can you say about how the energy separation between the ligand orbitals and the empty d orbitals changes as a function of the oxidation state of the transition metal at the center of the tetrahedral anion?
[23.84] The red color of ruby is due to the presence of Cr(III) ions at octahedral sites in the close-packed oxide lattice of Al2O3. Draw the crystal-field splitting diagram for Cr(III) in this environment. Suppose that the ruby crystal is subjected to high pressure. What do you predict for the variation in the wavelength of absorption of the ruby as a function of pressure? Explain.
23.85 In 2001, chemists at SUNY-Stony Brook succeeded in synthesizing the complex trans-[Fe(CN)4(CO)2]2–, which could be a model of complexes that may have played a role in the origin of life. (a) Sketch the structure of the complex. (b) The complex is isolated as a sodium salt. Write the complete name of this salt. (c) What is the oxidation state of Fe in this complex? How many d electrons are associated with the Fe in this complex? (d) Would you expect this complex to be high spin or low spin? Explain.
[23.86] When Alfred Werner was developing the field of coordination chemistry, it was argued by some that the optical activity he observed in the chiral complexes he had prepared was because of the presence of carbon atoms in the molecule. To disprove this argument, Werner synthesized a chiral complex of cobalt that had no carbon atoms in it, and he was able to resolve it into its enantiomers. Design a cobalt(III) complex that would be chiral if it could be synthesized and that contains no carbon atoms. (It may not be possible to synthesize the complex you design, but we won't worry about that for now.)
23.87 Generally speaking, for a given metal and ligand, the stability of a coordination compound is greater for the metal in the +3 rather than in the +2 oxidation state (for metals that form stable +3 ions in the first place). Suggest an explanation, keeping in mind the Lewis acid–base nature of the metal–ligand bond.
23.88 Many trace metal ions exist in the blood complexed with amino acids or small peptides. The anion of the amino acid glycine (gly),
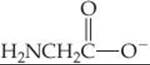
can act as a bidentate ligand, coordinating to the metal through nitrogen and oxygen atoms. How many isomers are possible for (a) [Zn(gly)2] (tetrahedral), (b) [Pt(gly)2] (square planar), (c) [Co(gly)3] (octahedral)? Sketch all possible isomers. Use the symbol ![]() to represent the ligand.
to represent the ligand.
[23.89] Suppose that a transition-metal ion was in a lattice in which it was in contact with just two nearby anions, located on opposite sides of the metal. Diagram the splitting of the metal d orbitals that would result from such a crystal field. Assuming a strong field, how many unpaired electrons would you expect for a metal ion with six d electrons? (Hint: Consider the linear axis to be the z-axis)
INTEGRATIVE EXERCISES
[23.90] Metallic elements are essential components of many important enzymes operating within our bodies. Carbonic anhydrase, which contains Zn2+ in its active site, is responsible for rapidly interconverting dissolved CO2 and bicarbonate ion, HCO3–. The zinc in carbonic anhydrase is tetrahedrally coordinated by three neutral nitrogen-containing groups and a water molecule. The coordinated water molecule has a pKa of 7.5, which is crucial for the enzyme's activity. (a) Draw the active site geometry for the Zn(II) center in carbonic anhy-drase, just writing “N” for the three neutral nitrogen ligands from the protein. (b) Compare the pKa of carbonic anhy-drase's active site with that of pure water; which species is more acidic? (c) When the coordinated water to the Zn(II) center in carbonic anhydrase is deprotonated, what ligands are bound to the Zn(II) center? Assume the three nitrogen ligands are unaffected. (d) The pKa of [Zn(H2O)6]2+ is 10. Suggest an explanation for the difference between this pKa and that of carbonic anhydrase. (e) Would you expect carbonic anhydrase to have a deep color, like hemoglobin and other metal-ion containing proteins do? Explain.
23.91 Two different compounds have the formulation CoBr(SO4) • 5 NH3. Compound A is dark violet, and compound B is red-violet. When compound A is treated with AgNO3(aq), no reaction occurs, whereas compound B reacts with AgNO3(aq) to form a white precipitate. When compound A is treated with BaCl2(aq), a white precipitate is formed, whereas compound B has no reaction with BaCl2(aq). (a) Is Co in the same oxidation state in these complexes? (b) Explain the reactivity of compounds A and B with AgNO3(aq) and BaCl2(aq). (c) Are compounds A and B isomers of one another? If so, which category from Figure 23.19 best describes the isomerism observed for these complexes? (d) Would compounds A and B be expected to be strong electrolytes, weak electrolytes, or nonelectrolytes?
23.92 A manganese complex formed from a solution containing potassium bromide and oxalate ion is purified and analyzed. It contains 10.0% Mn, 28.6% potassium, 8.8% carbon, and 29.2% bromine by mass. The remainder of the compound is oxygen. An aqueous solution of the complex has about the same electrical conductivity as an equimolar solution of K4[Fe(CN)6]. Write the formula of the compound, using brackets to denote the manganese and its coordination sphere.
23.93 The E° values for two low-spin iron complexes in acidic solution are as follows:
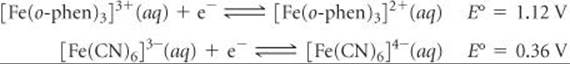
(a) Is it thermodynamically favorable to reduce both Fe(III) complexes to their Fe(II) analogs? Explain. (b) Which complex, [Fe(o-phen)3]3+ or [Fe(CN)6]3–, is more difficult to reduce? (c) Suggest an explanation for your answer to (b).
23.94 A palladium complex formed from a solution containing bromide ion and pyridine, C5H5N (a good electron-pair donor), is found on elemental analysis to contain 37.6% bromine, 28.3% carbon, 6.60% nitrogen, and 2.37% hydrogen by mass. The compound is slightly soluble in several organic solvents; its solutions in water or alcohol do not conduct electricity. It is found experimentally to have a zero dipole moment. Write the chemical formula, and indicate its probable structure.
23.95 (a) In early studies it was observed that when the complex [Co(NH3)4Br2]Br was placed in water, the electrical conductivity of a 0.05 M solution changed from an initial value of 191 ohm–1 to a final value of 374 ohm–1 over a period of an hour or so. Suggest an explanation for the observed results. (See Exercise 23.67 for relevant comparison data.) (b) Write a balanced chemical equation to describe the reaction. (c) A 500-mL solution is made up by dissolving 3.87 g of the complex. As soon as the solution is formed, and before any change in conductivity has occurred, a 25.00-mL portion of the solution is titrated with 0.0100 M AgNO3 solution. What volume of AgNO3 solution do you expect to be required to precipitate the free Br–(aq)? (d) Based on the response you gave to part (b), what volume of AgNO3 solution would be required to titrate a fresh 25.00-mL sample of [Co(NH3)4Br2]Br after all conductivity changes have occurred?
23.96 The total concentration of Ca2+ and Mg2+ in a sample of hard water was determined by titrating a 0.100-L sample of the water with a solution of EDTA4–. The EDTA4– chelates the two cations:
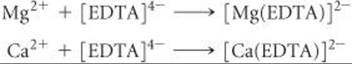
It requires 31.5 mL of 0.0104 M [EDTA]4– solution to reach the end point in the titration. A second 0.100-L sample was then treated with sulfate ion to precipitate Ca2+ as calcium sulfate. The Mg2+ was then titrated with 18.7 mL of 0.0104 M [EDTA]4–. Calculate the concentrations of Mg2+ and Ca2+ in the hard water in mg/L.
23.97 Carbon monoxide is toxic because it binds more strongly to the iron in hemoglobin (Hb) than does O2, as indicated by these approximate standard free-energy changes in blood:
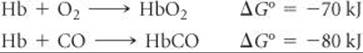
Using these data, estimate the equilibrium constant at 298 K for the equilibrium
![]()
[23.98] The molecule methylamine (CH3NH2) can act as a monoden-tate ligand. The following are equilibrium reactions and the thermochemical data at 298 K for reactions of methylamine and en with Cd2+(aq):
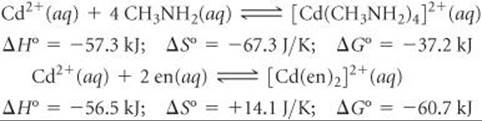
(a) Calculate ΔG° and the equilibrium constant K for the following ligand exchange reaction:
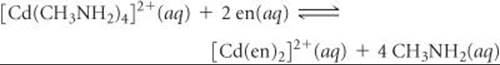
Based on the value of K in part (a), what would you conclude about this reaction? What concept is demonstrated? (b) Determine the magnitudes of the enthalpic (ΔH°) and the entropic (–TΔS°) contributions to ΔG° for the ligand exchange reaction. Explain the relative magnitudes.(c) Based on information in this exercise and in the “A Closer Look” box on the chelate effect, predict the sign of ΔH° for the following hypothetical reaction:

23.99 The value of Δ for the [CrF6]3– complex is 182 kJ/mol. Calculate the expected wavelength of the absorption corresponding to promotion of an electron from the lower-energy to the higher-energy d-orbital set in this complex. Should the complex absorb in the visible range?
[23.100] A Cu electrode is immersed in a solution that is 1.00 M in [Cu(NH3)4]2+ and 1.00 M in NH3. When the cathode is a standard hydrogen electrode, the emf of the cell is found to be +0.08 V. What is the formation constant for [Cu(NH3)4]2+?
[23.101] The complex [Ru(EDTA)(H2O)]– undergoes substitution reactions with several ligands, replacing the water molecule with the ligand. In all cases the ruthenium stays in the +3 oxidation state and the ligands use a nitrogen donor atom to bind to the metal.
[Ru(EDTA)(H2O)]– + L → [Ru(EDTA)L]– + H2O
The rate constants for several ligands are as follows:

(a) One possible mechanism for this substitution reaction is that the water molecule dissociates from the Ru(III) in the rate-determining step, and then the ligand L binds to Ru(III) in a rapid second step. A second possible mechanism is that L approaches the complex, begins to form a new bond to the Ru(III), and displaces the water molecule, all in a single concerted step. Which of these two mechanisms is more consistent with the data? Explain. (b) What do the results suggest about the relative donor ability of the nitrogens of the three ligands toward Ru(III)? (c)Assuming that the complexes are all low spin, how many unpaired electrons are in each?