Microreactors in Organic Chemistry and Catalysis, Second Edition (2013)
4. Automation in Microreactor Systems
4.5. Kinetic Model Discrimination and Parameter Fitting
Kinetic investigations are useful if the ultimate goal is to scale up from a bench-top process to a production scale. As microreactors have enhanced heat- and mass-transfer rates compared to more traditional batch procedures, they enable an intrinsic understanding of reaction kinetics [54]. These data may then be used to model reaction conversion at larger scale, where mixing may be less than ideal, reducing the time and materials required to find optimal production conditions. If the general form of the reaction rate law is not known a priori, experiments must first be run to discriminate between possible kinetic expressions, followed by fitting parameters to the most accurate model.
Typically, a number of experiments are run, and the resulting data are fit to potential models. However, this may result in a low level of information gained per experiment. A more efficient approach is to perform sequential experiments, analyzing the results between each set of conditions, and using the resulting models to determine the next set of conditions as those that provide the greatest information gain. In the Bayesian statistics approach, each model i has a probability pi of being correct, given by the formula
(4.5) 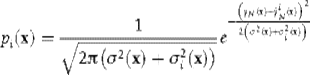

This approach experimentally was explored using the automated microfluidic system with automated HPLC sampling for a Diels–Alder reaction (Scheme 4.5). Three possible rate expressions were considered:
(4.6) 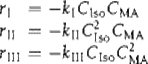
Scheme 4.5 Diels–Alder reaction [55, 56].
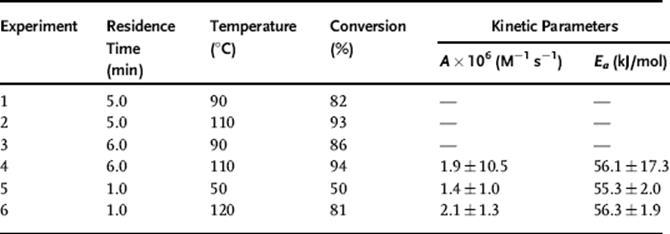
Temperature and residence time were varied in an initial half-factorial DoE to estimate the fitted reaction parameters. After these four experiments, subsequent experimental conditions were determined by the Bayesian approach, terminating once the Bayesian probability of one of the rate models passed 95%. The results from the fifth and sixth experiment are shown in Figure 4.8, which illustrates that after six experiments, only reaction model I matched with the experimental results, in agreement with the known rate law for the Diels–Alder reaction. Once the best rate law has been found, further experiments are performed to reduce the size of the joint confidence region of the fitted parameters using the d-optimal approach [57]. The results are summarized in Table 4.3, yielding an activation energy in agreement with the literature value of 58.5 ± 2 kJ/mol [5]. This reaction model was then used to predict conversion scaled up 500 times in a Corning Advanced Flow Reactor, yielding experimental results in good agreement with the predictions. It was then possible to perform a sensitivity analysis to understand what physical and chemical effects (e.g., dispersion, reaction kinetics, and heat transfer) had significant impact on the performance. In the present case, understanding the heat transfer characteristics was particularly important, which was consistent with the large activation energy for the reaction.
Figure 4.8 Probability distributions. (pi) of potential rate laws used for model discrimination calculations (a) before and (b) after d-optimal selection of experiment conditions. Experimental result is denoted by the arrow. Source: Reprinted with permission from the American Chemical Society [54].
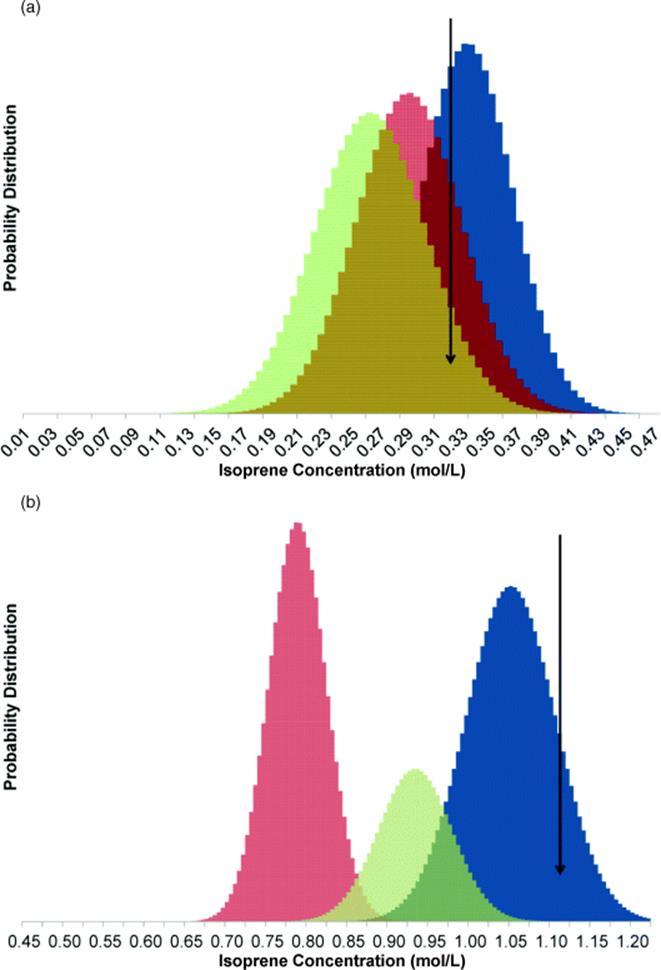
Table 4.3 Experimental conditions and resulting kinetic parameter 95% confidence intervals.
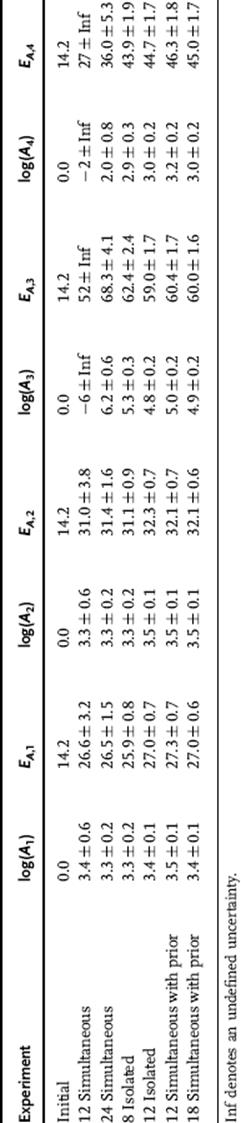
This approach was then applied to determine the kinetic parameters of a more complex network of reactions, the nucleophilic aromatic substitution (SNAr) reaction of 2,4-dichloropyrimidine with morpholine (Scheme 4.6), where the uncertainties in the kinetic parameters are more intrinsically coupled [58]. Assuming that all four reactions exhibit second-order kinetics, the activation energies and pre-exponential factors were determined in a three-step process. First, an initial set of experiments was run to estimate the eight kinetic parameters simultaneously. Then, each reaction was examined individually. Lastly, these estimates were used as the prior for a final estimate of all the parameters in unison. These experiments varied temperature, residence time, benzyl alcohol concentration, and morpholine equivalents. The results from these experiments are summarized in Table 4.4. The initial simultaneous parameter estimates resulted in large uncertainties in the activation energies EA,3 and EA,4 due to interconnection of the variables and the low sensitivity of these two parameters. These uncertainties were significantly reduced by studying the reactions in isolation to decouple the variables and using these values as the priors for the final simultaneous d-optimal parameter fitting.
Scheme 4.6 Multistep reaction network for conversion of 2,4-dichloropyrimidine to 4,4′-(2,4-pyrimidinediyl)bis-morpholine [5].
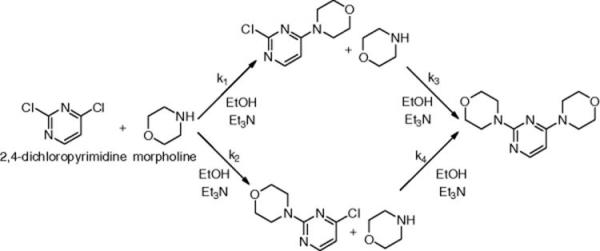
Table 4.4 Optimal kinetic parameter estimates (Ai in M−1 s−1 and EA,i in kJ/mol) and uncertainties (1 standard deviation)