Botany: An Introduction to Plant Biology - Mauseth, James D. 2017
Genetics and Evolution
Seed Plants I: Seed Plants Without Flowers (“Gymnosperms”)

Chapter Opener Image: At this stage, these cones of a pine tree (Pinus) have been pollinated and seeds are developing inside but they are not quite mature enough to be released. These cones are the equivalent of unripe fruits in flowering plants. When these cones were pollinated, they were much smaller, about one centimeter long, and the scales were separated from each other so that wind-borne pollen could just settle into the ovules between the cone scales. When these cones are mature, they will be dry, tough, and inedible, like the fruits of many angiosperms. Fir cones fall apart when they are mature, and the seeds simply fall to the ground; in other conifers, the cones expand and drop their seeds, and in still others they open only when heated by a fire. It is very rare for any conifer cone to be fleshy and edible, but those of junipers (Juniperus) are.
OUTLINE
✵ Concepts
✵ Division Progymnospermophyta: Progymnosperms
- Aneurophytales
- Archaeopteridales
- Evolution of Seeds
✵ Division Pteridospermophyta: Seed Ferns
✵ Division Coniferophyta: Conifers
✵ Division Cycadophyta: Cycads
✵ Division Cycadeoidophyta: Cycadeoids
✵ Division Ginkgophyta: Maidenhair Tree
✵ Division Gnetophyta
LEARNING OBJECTIVES
After reading this chapter, students will be able to:
✵ Explain the process that led to the evolution of seeds.
✵ Describe the features of progymnosperms.
✵ Name two orders in the division Progymnospermophyta.
✵ Identify three features of seed ferns.
✵ Recall four features of conifers.
✵ Compare seed cones to pollen cones.
✵ Explain how cycads differ from seed ferns.
✵ List five features of the division Ginkgophyta.
✵ Name the three plant groups in the division Gnetophyta.
 Did You Know?
Did You Know?
✵ Most of the plants in this chapter are conifers: Christmas trees and their relatives.
✵ All species in this chapter have two types of cones: pollen cones and seed cones.
✵ Before the evolution of seeds, animals had only leaves, stems, and roots to eat, all of which are much lower in nutrients than seeds.
✵ The plants in this chapter are usually called gymnosperms but recent evidence indicates these plants are not one complete clade.
![]() Concepts
Concepts
The life cycle of vascular cryptogams is an alternation of independent, heteromorphic generations. A disadvantage of this life cycle is that the new sporophyte, while developing from the zygote, is temporarily dependent on a tiny gametophyte for its start in life. Consequently, many new sporophytes perish. All of the genes of each gametophyte and half the genes of each new sporophyte embryo are identical to those of the maternal sporophyte, and thus, any mutation that improves the survival of the gametophytes or embryos contributes to the reproductive success of the maternal sporophyte. It would be advantageous if the embryo could use the photosynthetic and absorptive capacity of the leaves and roots of the previous sporophyte. In order for this to happen, the megagametophyte and embryo must be retained inside the maternal sporophyte; this is accomplished by retaining the megaspore and allowing the megagametophyte to develop within the sporangium (FIGURES 22-1, A—D). Such an arrangement requires some alteration in the microgametophyte as well because retention of the megagametophyte changes its position. Free-living gametophytes develop on the soil, whereas retained ones develop high on the plant in strobili, a position that cannot be reached by swimming sperm cells produced by microgametophytes living at the soil surface. The modifications that overcame this problem are simple: The megasporophylls with the megagametophytes were arranged in upright cone-like structures, allowing microspores to be carried by wind and dropped into the megasporangiate cone (FIGURE 22-1E). There they would germinate into a microgametophyte, produce their antheridia and sperm cells, and carry out fertilization. Fertilization itself has changed little, with a moist sporophyll replacing moist soil. These changes produced the first seed plants, the division Pteridospermophyta or seed ferns (the “p” in “pterido” is silent: “terido sperm AH fit a”) and the early members of division Coniferophyta, known as class Cordaitales. These two groups are now extinct, but their descendants still exist as the living seed plants. In several lines of evolution, those that produced the cycads and Ginkgo, this method of fertilization still exists, and beautiful swimming sperms are produced. In other lines, those leading to conifers and to flowering plants, sperms have become nonmotile and are carried to the egg by growth of the microgametophyte as a pollen tube.
Evolution of seeds was preceded by evolution of a vascular cambium. Vascular cambia had arisen independently in some lycophytes and equisetophytes, but cells in this new cambium could undergo radial longitudinal divisions, thus allowing the cambium to grow in circumference as wood accumulated. In contrast to the rather slender lycophyte and equisetophyte trees, this new type of cambium could—and still does—produce trees as massive as giant redwoods, oaks, and hickories. We believe that this cambium arose just once, in one group of plants that then gave rise to a monophyletic group of woody plants, the lignophytes (FIGURE 22-2). Shortly afterward, seeds originated, establishing the seed plants, spermatophytes. The plants that existed after the origin of wood but before that of seeds were trees that reproduced with spores, the way ferns reproduce.
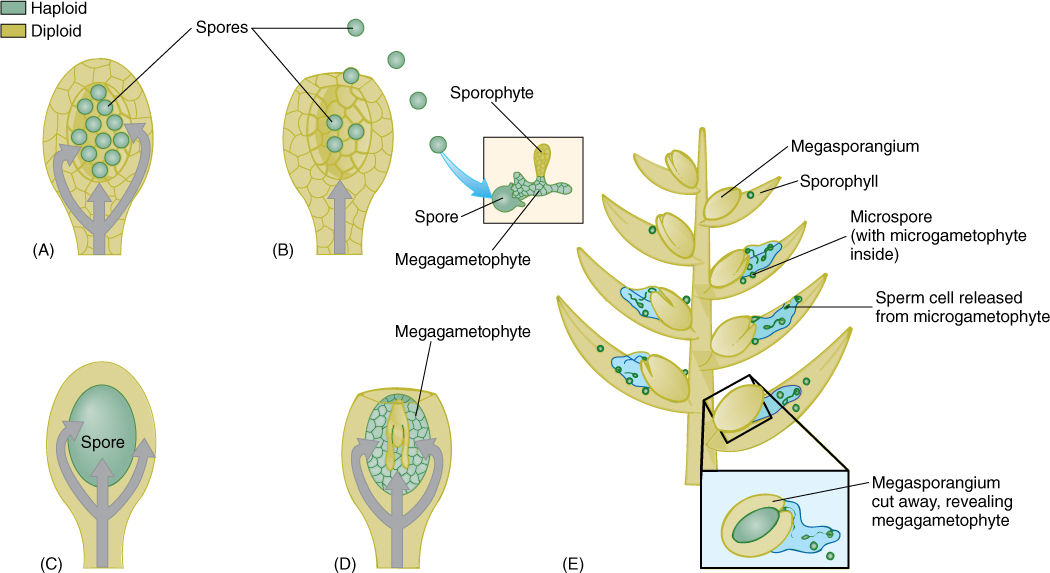
FIGURE 22-1 (A) and (B) Plants that release megaspores. Only limited amounts of nutrient can be placed in a spore, and after spore release (B), the sporophyte can do nothing more to nourish or protect the gametophytes or new sporophytes. (C) and (D) Plants that retain megaspores. Mutations that cause a reduction in the number of megaspores and their retention in the sporangium can be selectively advantageous. The sporophyte can nourish and protect its gametophytes and also the subsequent progeny sporophytes, at least for a while. In (D), the embryo is shown embedded in tissue of the megagametophyte; this occurs in gymnosperms—seed plants that are not flowering plants. In flowering plants, the tiny megagametophyte is quickly replaced by endosperm. (E) Megasporangia that are packed together in a cone composed of sporophylls automatically have a means of catching and retaining microspores. These germinate into microgametophytes with antheridia that release sperms within a millimeter or two of the megagametophyte and its archegonia.
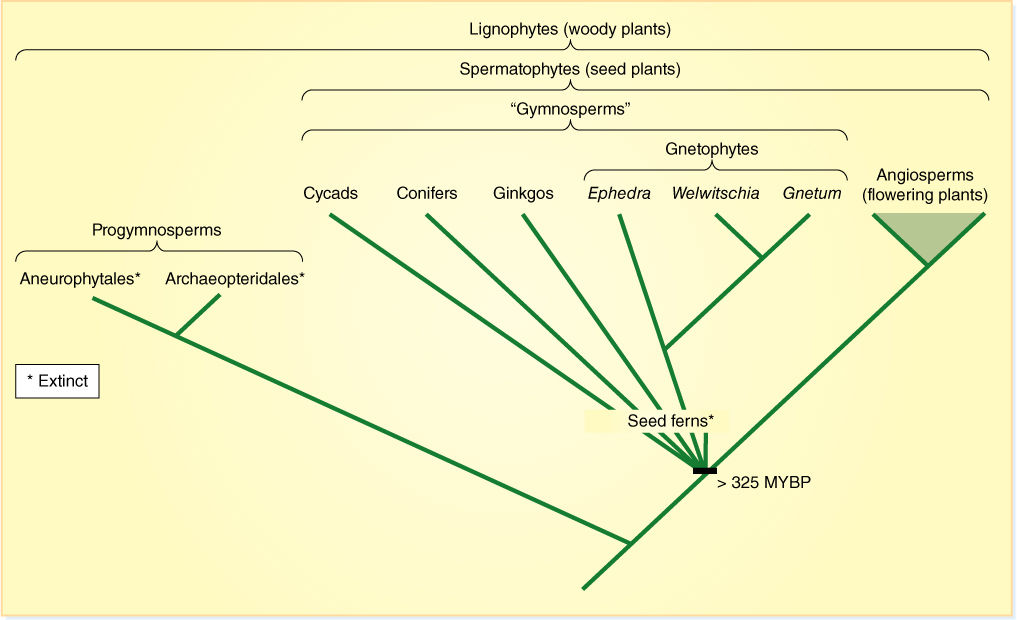
FIGURE 22-2 One recently proposed cladogram of seed plants (MYBP = million years before present).
We are still not certain of the exact evolutionary relationships of all groups of extant seed plants and their extinct ancestors. Notice that in Figure 22-2, many groups emerge from the same point of the cladogram; this is an unresolved polychotomy, and it just means that we do not have enough information to know the order in which the various groups and their innovations arose. One problem is that we cannot extract and analyze DNA from such long-dead fossils.
As you read the following descriptions, keep in mind that certain features are often associated with certain others: There appear to be two major suites of characters. Some plants produce a small amount of very soft, spongy, parenchymatous wood (manoxylic wood, see Figure 22-26), have large, compound leaves and radially symmetrical seeds. This suite occurs in cycads and their relatives. The second suite consists of hard, strong wood with little parenchyma (pycnoxylic wood; FIGURE 22-3), small, simple leaves and flattened seeds (bidirectionally symmetrical). These occur in conifers and their relatives. The coniferophyte syndrome (and plants) probably evolved out of the cycadophyte syndrome, but as Figure 22-2 shows, we are not yet certain of this.
An old classification from the 1800s grouped all the seed plants together in a single division, Spermatophyta, with two classes, class Gymnospermae and class Angiospermae (Figure 22-2). The gymnosperms are those plants with “naked ovules,” that is, ovules located on flat sporophylls, for example, pine cones. Angiosperms are the flowering plants, those with carpels, which are believed to be sporophylls that form a tube-like, closed structure; fruits are mature carpels. This classification emphasized both the close relationship of all seed plants and the idea that angiosperms originated from some group of gymnosperms, but if angiosperms evolved from some type of gymnosperm, then the group “gymnosperms” is not natural because it leaves out some of the descendants (angiosperms) of the ancestors. In cladistic terms, an incomplete group is a paraphyletic group. Consequently, class “Gymnospermae” should be abandoned.
The divisions of living seed plants commonly accepted now and used in this book are (1) division Cycadophyta, (2) division Coniferophyta, (3) division Ginkgophyta, (4) division Gnetophyta (these four are gymnosperms), and (5) division Magnoliophyta (the flowering plants). Numerous extinct groups exist, the most significant for us being progymnosperms and seed ferns.
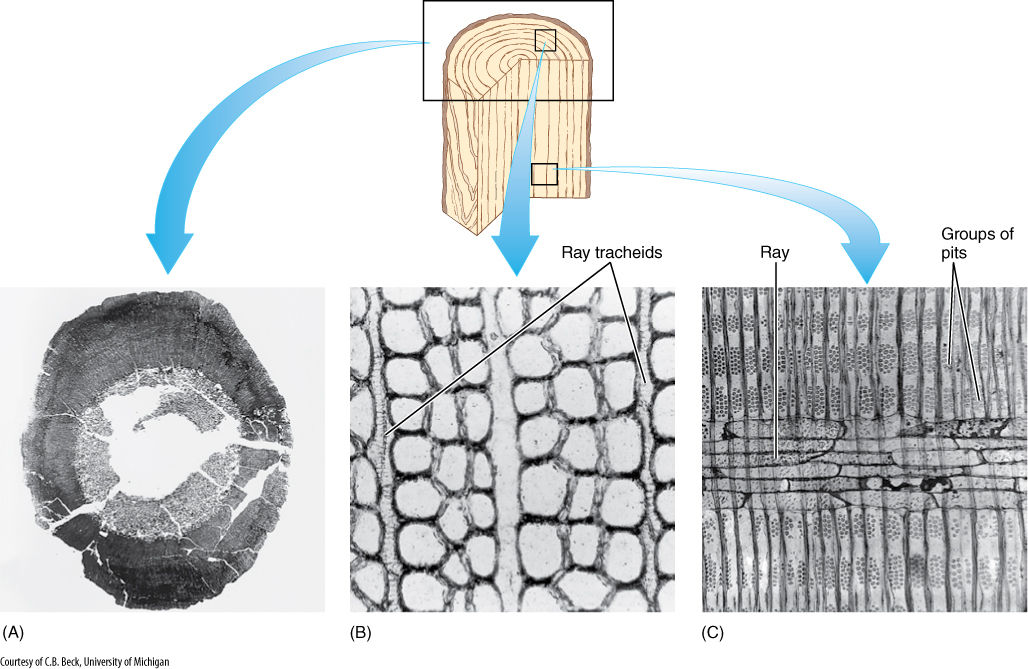
FIGURE 22-3 (A) The vascular cambium of progymnosperms was able to produce a virtually unlimited amount of both secondary xylem and secondary phloem, as in this Callixylon brownii. (B) and (C) Progymnosperm wood had large vertical tracheids with pitted walls and an abundance of rays with ray tracheids. Ray tracheids do not occur in flowering plants but are common in conifers as horizontal tracheids that permit rapid conduction horizontally in the sapwood. (B) C. erianum (× 700). (C) C. newberryi (× 375).
![]() Division Progymnospermophyta: Progymnosperms
Division Progymnospermophyta: Progymnosperms
Trimerophytes were an important group of plants that evolved from rhyniophytes. One evolutionary trend initiated in trimerophytes was pseudomonopodial branching and the first steps in the evolution of megaphylls (euphylls). These evolutionary trends were continued in horsetails and ferns, which evolved out of trimerophytes.
A third group to evolve from trimerophytes was the now-extinct progymnosperms (TABLE 22-1) so named because some gave rise later to conifers, cycads, and the other gymnosperms. Like ferns and horsetails, progymnosperms also developed megaphyllous leaves; however, another feature was just as significant—the evolution of a vascular cambium with unlimited growth potential and capable of producing both secondary xylem and secondary phloem.
As early as 360 million years ago, the vascular cambium that evolved in progymnosperms was capable of undergoing radial longitudinal divisions, and thus, it could function indefinitely, producing large amounts of both secondary xylem and phloem (Figures 22-3 and FIGURE 22-4). The wood was almost indistinguishable from that of many living conifers. It contained elongate tracheids, most with circular bordered pits. It had little or no axial parenchyma. Rays were tall and uniseriate and consisted of procumbent ray tracheids. This wood must have been strong, effective at conduction, and capable of supporting a large mass of leaves and branches. At least some species, Triloboxylon and Proteokalon, for example, had a cork cambium that produced bark. Progymnosperms produced true woody trees: Trunks were up to 1.5 m in diameter and 12 m tall.
TABLE 22-1 Classification of Division Progymnospermophyta*
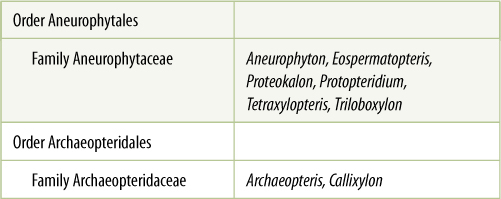
*All species of this group are extinct.
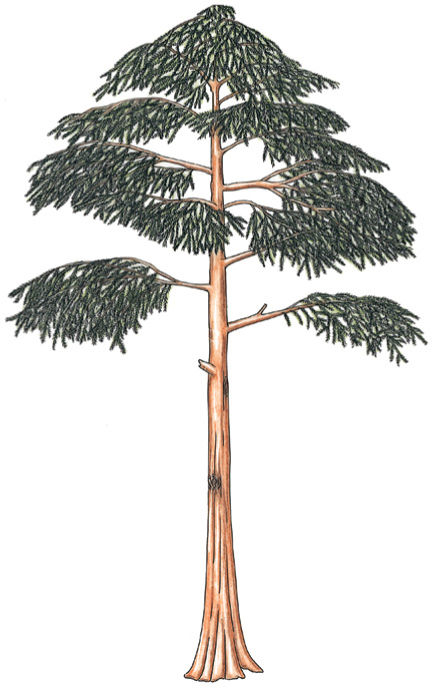
FIGURE 22-4 Reconstruction of Archaeopteris, a small tree about 6 m tall. Although extinct now, Archaeopteris flourished about 360 million years ago across northern North America, Canada, and Europe.
Although progymnosperm wood was similar to that of conifers, the two groups must be kept separate because progymnosperms did not have seeds or even ovule precursors. The species that arose later were heterosporous, but megaspores were shed through longitudinal slits in the sporangia; they were not retained in an indehiscent sporangium as occurs in seed plants. Although leaves and wood of progymnosperms were quite advanced, their reproduction was remarkably simple.
Aneurophytales
The order Aneurophytales contains the more relictual progymnosperms, such as Aneurophyton, Protopteridium, Proteokalon, Tetraxylopteris, Triloboxylon, and Eospermatopteris (FIGURE 22-5). They varied in stature from shrubs (Protopteridium, Tetraxylopteris) to large trees, up to 12 m tall. They all had a vascular cambium and secondary growth, but the primary xylem of their stems was a protostele like that of rhyniophytes and trimerophytes. Aneurophytes further resembled trimerophytes in having little webbing between their ultimate branches; these could not yet be called leaves.
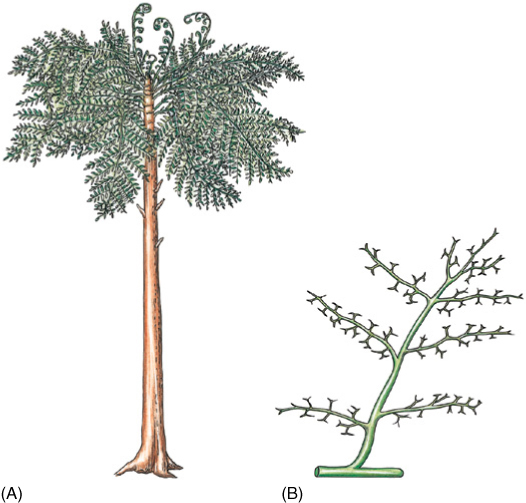
FIGURE 22-5 (A) Eospermatopteris was a member of the Aneurophytales; it had a trunk and what appear to be frond-like leaves, but these were just branch systems. Planation and webbing had not yet evolved. (B) A portion of the “leaf” of Aneurophyton.
Archaeopteridales
A more derived progymnosperm was Archaeopteris in the order Archaeopteridales. These were trees up to 8.4 m tall with abundant wood and secondary phloem (Figure 22-4). Stems of Archaeopteris had a siphonostele, pith surrounded by a ring of primary xylem bundles, much like modern conifers and dicots. Although the “fronds” of archaeopterids resembled fern leaves, close examination reveals that they were actually planated branch systems and that the ultimate “leaflets” were really spirally arranged simple leaves. Webbing was only partial in A. macilenta (FIGURE 22-6), but it is complete in A. halliana and A. obtusa; these can be considered full-fledged megaphylls.
Reproduction in archaeopterids was heterosporous. Megaspores measured up to 300 mm in diameter and microspores only 30 mm in diameter, and each type was produced in its own distinctive sporangium—broad megasporangia and slender microsporangia. Sporangia were terminal on short branches mixed with sterile, leaf-like branch systems. Megaspores were released from the sporangia, not retained. Seeds were not produced.
Evolution of Seeds
Investigating the life cycle of extinct plants based on fossil material is difficult, but not impossible. In free-sporing species, spores can be identified with sporophytes if some spores were trapped in a sporangium attached to leaves or wood during fossilization. Unfortunately, spores cannot be identified with gametophytes except when the gametophyte is microscopic and develops within the spore wall. If megaspores are retained in the megasporangium, at least some of the investigation is easier.
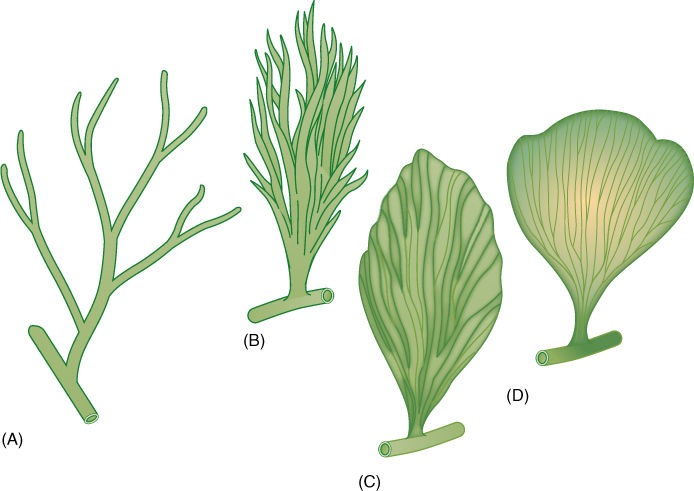
FIGURE 22-6 During the existence of the plants classified in the extinct genus Archaeopteris, the final transitions from telomes to leaves occurred. (A) Archaeopteris fissilis. (B) A. macilenta. (C) A. halliana. (D) A. obtusa. What appear to be veins in the leaves were actually the shoot axes of the telomes.
Currently, the earliest known progymnosperm species with heterospory is Chauleria from the Middle Devonian Period, approximately 390 million years ago. In an Upper Devonian fossil, Archaeosperma arnoldii, the megasporangium produced only one megaspore mother cell, and this produced only one large, viable megaspore and three small, aborted cells. The megasporangium was surrounded by a layer of tissue, an integument, that projected upward. There was a large micropyle, a hole in the integument that permitted the sperm cells to swim to the egg after the megaspore had developed into a megagametophyte and had produced eggs (FIGURE 22-7). This is similar to angiosperm ovules, and some fossils help us understand the early stages. In Genomosperma kidstoni, the megasporangium was closely surrounded by sterile telomes (FIGURE 22-8). In G. latens, the telomes were fused at the base, and in Eurystoma angulare, they were similar to Archaeosperma, fused into one structure except at the tip. The sheath of sterile branches must have been important in trapping wind-blown microspores, but at first it would have allowed them to settle anywhere on the megasporangium. As they fused to each other and to the megasporangium, the space at the top of the megasporangium became the place where microspores settled, acting as a pollen chamber or holding area. As megasporangia evolved into ovules with integuments, other telomes on nearby branches became modified into cupules (Figure 22-7A), which may have later given rise to the carpel in angiosperms.
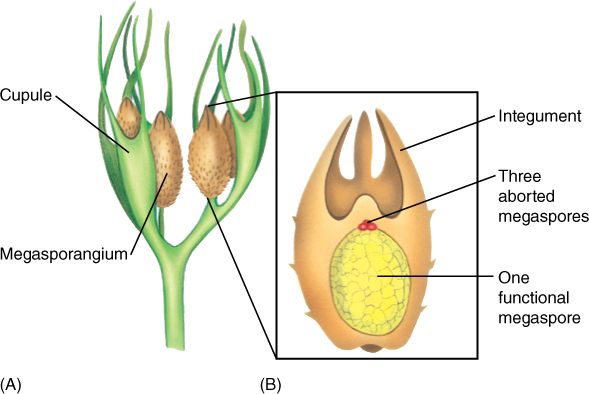
FIGURE 22-7 A reconstruction of the megasporangium (B) and adjacent telomes (A) of Archaeosperma arnoldii. Each sporangium contained only one large megaspore; the other three products of the spore mother cell meiosis apparently degenerated early. Tissue immediately around the megaspore was the megasporangium wall. Attached to it and extending upward as finger-like projections was a layer called an integument. Above the sporangium was a space surrounded by integument tissue, a calm, wind-free pollen chamber where pollen or spores settled. Around each megasporangium and integument was another set of partially fused telomes.
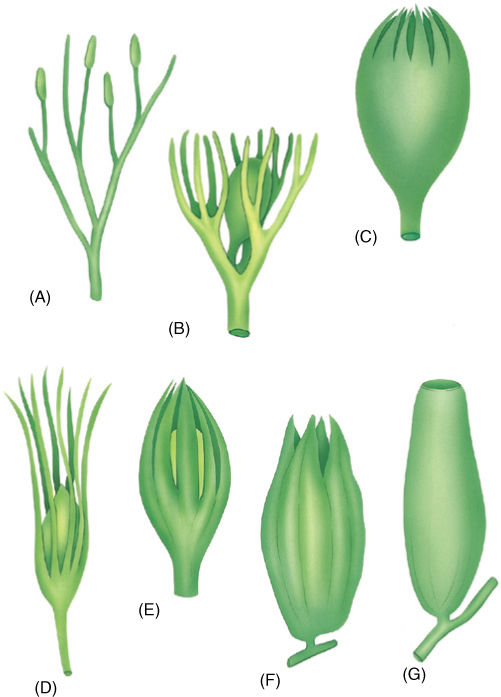
FIGURE 22-8 (A) — (C) A hypothetical evolution of an integument from telomes. In (A), sporangia are neither clustered nor particularly associated with sterile telomes. (B) One megasporangium is surrounded by sterile telomes. (C) The sterile telomes have fused at their bases. (D) — (G) Actual fossils that correspond to the hypothesis. (D) Genomosperma kidstoni. (E) G. latens. (F) Eurystoma angulare. (G) Stamnostoma hyttonense.
Simultaneously, microspores were evolving into pollen grains. Changes occurred in the nature of their wall, in their method of germination, and in the nature of the microgametophyte they produced. By the Carboniferous Period, just after the Upper Devonian Period (approximately 340 million years ago; see the timeline on the inside of the back cover), four types of pollen had become common. In some, internal structure is preserved well enough to see that these grains already had internal microgametophytes remarkably similar to those of modern gymnosperms.
![]() Division Pteridospermophyta: Seed Ferns
Division Pteridospermophyta: Seed Ferns
Progymnosperms gave rise to another line of gymnospermous plants in addition to the conifers: the cycadophytes (see Figure 22-2). These are classified as three divisions: Pteridospermophyta (seed ferns, all extinct), Cycadophyta (cycads, extant), and Cycadeoidophyta (cycadeoids, all extinct).

FIGURE 22-9 Reconstruction of a swamp forest during the Carboniferous Period. The large trees are related to Lepidodendron (Sigillaria rugosa on the left, S. saulli on the right), and the smaller fern-like plants are seed ferns (Neuropteris decipiens), not true ferns. Note the seeds at the ends of some fronds.
The earliest seed ferns appeared in the Upper Devonian Period—others appeared later. Not all are closely related to each other, they form a grade (a level of evolution) rather than a clade (all of the descendants of a common ancestor, regardless of their level of evolution): Seed ferns were any woody plant with fern-like foliage that bore seeds instead of sori (clusters of sporangia) on its leaves. Many resembled modern tree ferns (except they had wood), others were vines (FIGURE 22-9).
Pteridosperms are thought to have evolved from the Aneurophytales because the earliest seed ferns, such as Stenomyelon, had a three-ribbed protostele as in the aneurophytes Triloboxylon and Aneurophyton. In later species of Stenomyelon, Calamopitys, and Lyginopteris, most central cells of the stem differentiated as parenchyma, not tracheids. They had a ring of vascular bundles surrounding a pith, an arrangement that also occurs in the stems of all gymnosperms and angiosperms. Seed ferns had a long-lived vascular cambium that produced both xylem and phloem; this too is similar to gymnosperms and angiosperms. Although their wood was basically similar to that of their progymnosperm ancestors, there were interesting differences. Tracheids were much longer and wide enough that several rows of circular bordered pits could occur on each radial wall; conifer tracheids are so narrow that only one or two rows can fit on each radial wall. Rays in pteridosperm wood were many cells wide, not just one cell wide, and they were very tall, being large wedges of parenchyma: Seed fern wood was manoxylic, much softer and less dense than wood of conifers and progymnosperms, and manoxylic wood also occurs in cycads (FIGURE 22-10) and cycadeoids. Around the stem of pteridosperms was a thick cortex that contained distinctive radial plates of sclerenchyma just below the epidermis. The inner cortex contained secretory ducts. In older plants, a cork cambium and bark formed exterior to the secondary phloem; the cortex was shed with the first bark.
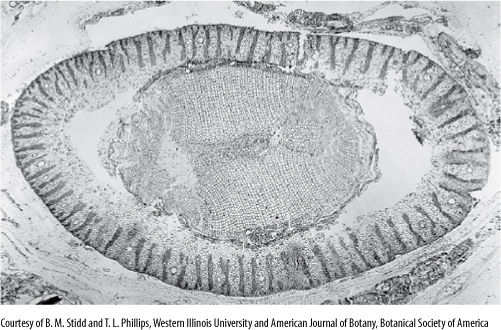
FIGURE 22-10 Transverse section of Schopfiastrum stem, showing the beginning of a pith, parenchymatous wood, and the distinctive cortex with plates of sclerenchyma (× 10).
Leaves of seed ferns were similar to those of true ferns in overall organization—large, compound, and planar. Unlike ferns, however, the foliage leaves of seed ferns bore seeds (FIGURE 22-11). Within the seed fern ovule, the megasporangium (nucellus) was large, and bundles of vascular tissue ran into and through it. The integument was attached to the megasporangium only at the base and was vascularized. Seeds could be extremely large, up to 11 cm long and 6 cm in diameter in the now extinct Pachytesta incrassata.
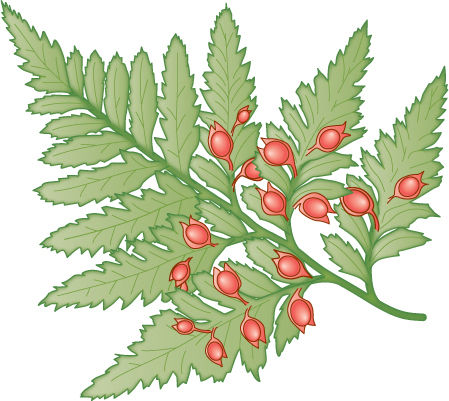
FIGURE 22-11 Seed ferns, such as this Emplectopteris, bore seeds along their leaves, not in cones. Otherwise, seed fern leaves were remarkably analogous (not homologous) to the leaves of true ferns in terms of venation and tissue structure.
![]() Division Coniferophyta: Conifers
Division Coniferophyta: Conifers
Because conifers are familiar plants, they will be discussed first (FIGURE 22-12 and TABLE 22-2). They are diverse (approximately 50 genera and 550 species), and all are trees of moderate to gigantic size; the giant redwoods of California (Sequoiadendron giganteum) reach 90 m in height and 10 m in diameter (10 meters is the distance on a football field from the goal line to the 30 yard line, and this is trunk diameter). Conifers are never vines, herbs, or annuals, and they never have bulbs or rhizomes. Conifer leaves are always simple needles or scales. Leaves of most conifers are perennial, persisting for many years (FIGURE 22-13); leaves of Agathis and Araucaria remain even on very old trunks.
The venation of conifer leaves is often simple, with just one or two long veins running down the center of a needle-shaped leaf or several parallel veins in scale-shaped leaves. Leaf veins have an endodermis and a tissue called transfusion tissue, consisting of transfusion parenchyma cells and transfusion tracheids (FIGURE 22-14). The latter are more or less cuboidal and have prominent circular bordered pits. Transfusion parenchyma is intermixed with the tracheids, the two cell types forming a complex three-dimensional pattern that facilitates transfer of materials between the ordinary vascular tissues and the mesophyll tissue outside the endodermis.
TABLE 22-2 Classification of Division Coniferophyta
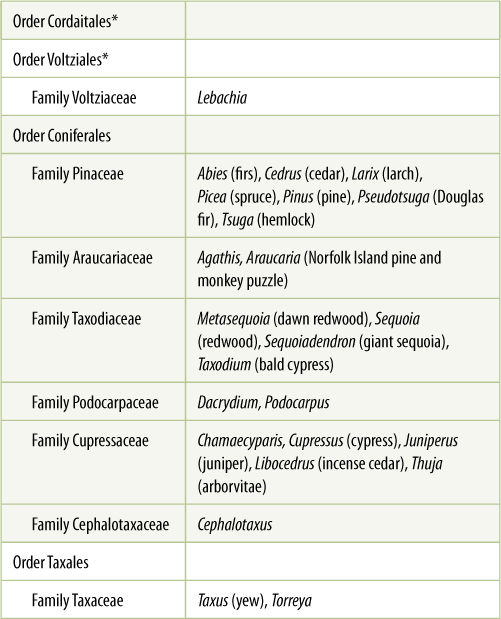
*All species of this group are extinct.

FIGURE 22-12 Division Coniferophyta is large and diverse, containing many familiar plants. (A) Pine (Pinus). (B) European larch (Larix decidua). (C) Big tree redwood (Sequoiadendron giganteum). (D) Western juniper (Juniperus occidentalis).

FIGURE 22-13 Conifer leaves are never compound. They are always simple and leathery, needle-like in some, and scale-like in others. (A) In bristlecone fir (Abies bracteata), each leaf has two white bands where stomata are located. (B) Scale-like leaves of incense cedar (Calocedrus decurrens). (C) Pines have two types of leaves—small, papery brown scales on the long shoots (visible on the branch) and the long needles borne by the short shoots located in the axils of the papery scale leaves. The long needles were produced last year.
Just as in their progymnosperm ancestor Archaeopteris, wood of modern conifers lacks vessels, and their phloem lacks sieve tubes. Tracheids are so narrow that only one or two rows of circular bordered pits occur on their radial walls. All conifers have pollen cones and seed cones, most of which are woody, but in Juniperus and Podocarpus, seed cones superficially resemble the fruits of flowering plants. The conifers are still a very successful group, forming extensive forests covering over 17 million km2; in many of these forests, flowering plants exist only as herbs, shrubs, or small trees growing in the conifers’ shade.
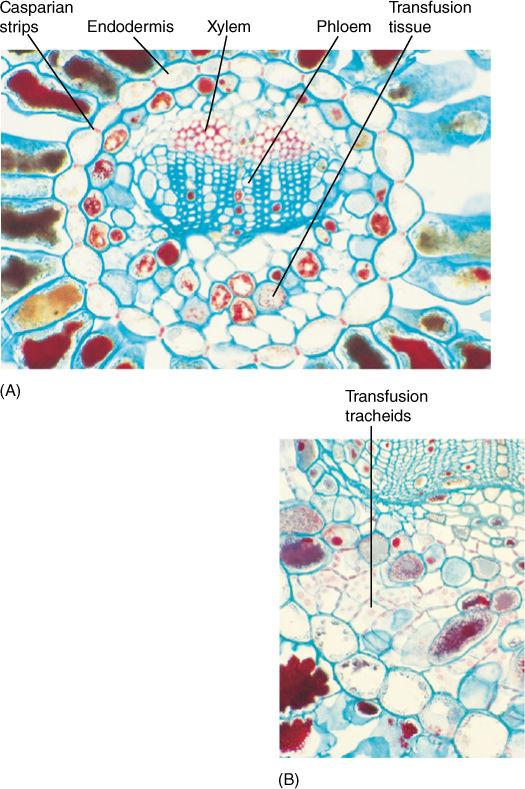
FIGURE 22-14 (A) The vascular bundle of this Douglas fir leaf has an endodermis, xylem, phloem, and transfusion tissue (× 80). (B) High magnification of a pine leaf bundle showing secondary phloem and transfusion tracheids. Casparian strips are visible in the endodermis walls (× 130).
The pines are good representatives for closer examination. The trees are monopodial, with one main trunk bearing many branches; the wood is composed exclusively of tracheids, but annual rings, spring wood, and summer wood are all visible because large-diameter tracheids are produced in the spring, followed by narrow-diameter tracheids in the summer (FIGURE 22-15). Rays are thin and tall and contain both ray parenchyma and ray tracheids. Resin canals, which produce the thick, sticky pitch, run vertically among the tracheids and horizontally in the rays. The wood has almost no axial parenchyma. Phloem contains sieve cells and albuminous cells and also has tall, narrow rays (FIGURE 22-16). A cork cambium produces a thick, tough bark that provides excellent protection even from forest fires.
Pines, like several other conifers, have two types of shoot, each with a characteristic type of leaf. Tiny papery leaves occur on long shoots and in their axils are short shoots that produce the familiar long needle leaves (see Figure 22-13C and FIGURE 22-17). The leaves have many xeromorphic characters: thick cuticle, sunken stomata, cylindrical shape.
Like all conifers, pines have both pollen cones and seed cones. Pollen cones are simple cones with a single short unbranched axis that bears microsporophylls (FIGURE 22-18). Microspore mother cells undergo meiosis and form microspores; then each of these develops endosporially into a small gametophyte with four cells, one of which is a generative cell as in flowering plants (FIGURE 22-19 and FIGURE 22-20). The gametophytes are shed from the tree as pollen and carried by wind; a small percentage land in seed cones, but the great majority land elsewhere and die.
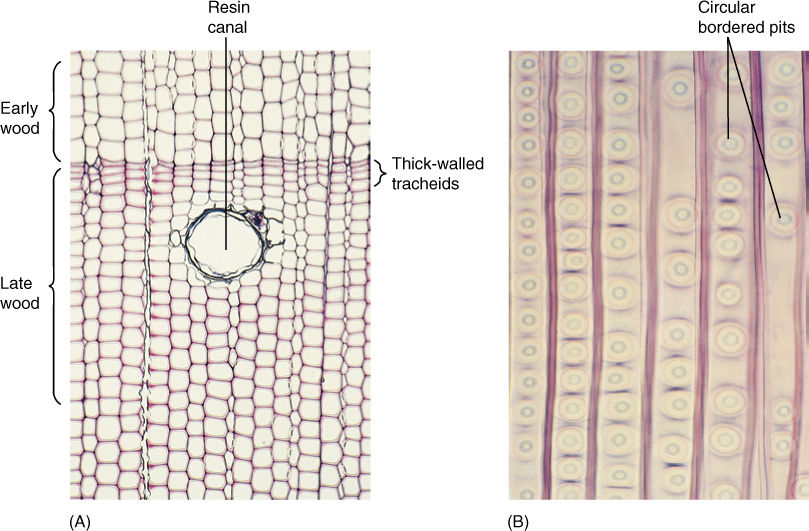
FIGURE 22-15 (A) Wood of pine, like that of all conifers, lacks vessels; it consists almost exclusively of tracheids. Growth rings are visible because late wood contains narrow, thick-walled tracheids, whereas early wood contains wide, thin-walled tracheids (transverse section, 330). (B) Pine tracheids are narrow and their circular bordered pits are wide, and thus, only one row of pits fits on a given wall, unlike the dozens of small pits that cover each wall of an angiosperm vessel (× 80).
Seed cones are more complex than pollen cones: They are compound cones, each consisting of a shoot with axillary buds. The short axis bears leaves called cone bracts rather than sporophylls (FIGURE 22-21). Each bract has an axillary bud that bears the megasporophylls. In some fossil conifers, the individual structures can still be seen, but in all modern conifers, extensive fusion has occurred: The axillary bud is microscopic, and its megasporophylls are fused laterally, forming an ovuliferous scale (FIGURE 22-22). In larch, fir, and Douglas fir (Pseudotsuga), the ovuliferous scale is still distinct and can be seen, but in most conifers, it is fused to the bract.
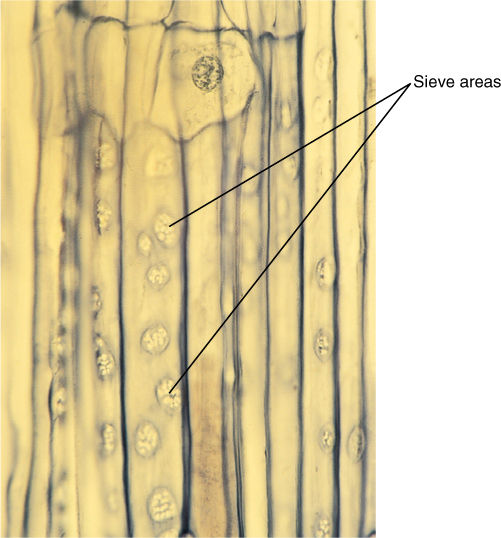
FIGURE 22-16 Phloem of conifers contains sieve cells, not sieve tube members; sieve cells are long and narrow with sieve areas over much of their surface, but they never have horizontal cross walls (sieve plates) with enlarged sieve pores. The sieve areas in this pine are particularly abundant and easy to see (× 25).

FIGURE 22-17 In Cedrus atlantica, the nature of short shoots is obvious because they form more needles each year and so slowly grow into a visible shoot.
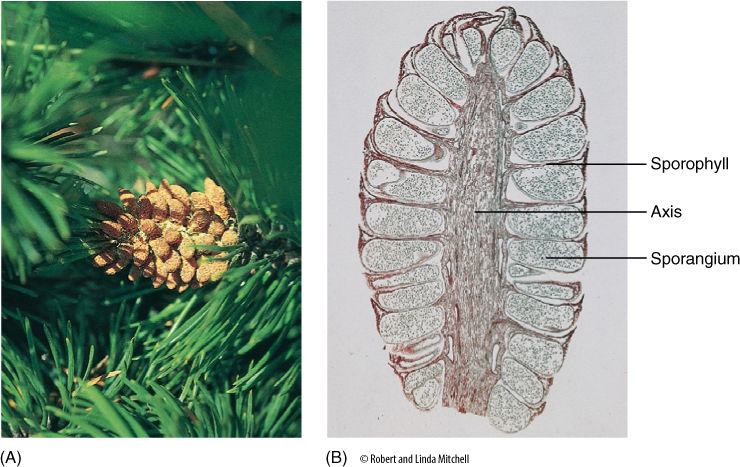
FIGURE 22-18 (A) Pollen cones typically occur in clusters near the ends of branches. As the microsporangia dehisce, pollen is liberated to the wind and blown away for distribution. Such a method of gene transfer is inefficient because so few pollen grains land on seed cones; it is successful primarily because conifers grow as dense forests, where each conifer is surrounded by hundreds of potential partners. (B) Pollen cones are simple cones; they have one single stem axis and bear microsporophylls. These are not microsporangia but rather leaf-like structures that bear microsporangia (× 10).
Inside each megasporangium, a single large megaspore mother cell undergoes meiosis, with three of the resulting cells degenerating and only one surviving as the megaspore (FIGURE 22-23). The megasporangium does not dehisce; the megaspore is retained inside and grows into a large coenocytic (multinucleate) megagametophyte by free nuclear divisions and may have as many as 7,200 nuclei. It is much larger and more complex than the simple embryo sac of angiosperms. Development can take as long as a year, but finally walls form, converting the coenocyte into a cellular megagametophyte. Two or three archegonia form as sets of cells, each surrounding a large egg (FIGURE 22-24). Conifer eggs are gigantic cells loaded with carbohydrate and protein. The egg nucleus, although haploid, is swollen to a volume much larger than that typical for entire cells. It is probably filled with DNA synthetases and RNA polymerases, ready for extremely rapid activity once karyogamy with a sperm nucleus occurs.
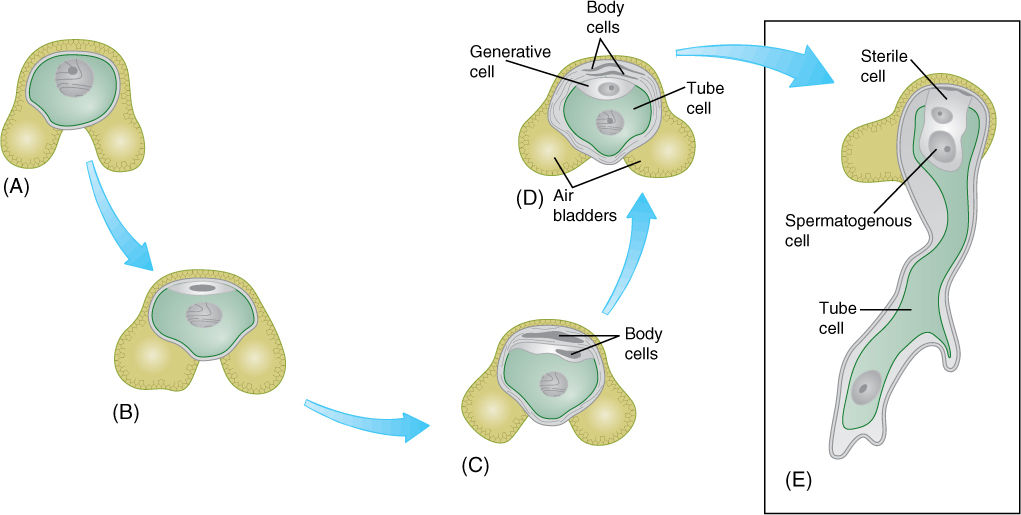
FIGURE 22-19 (A) and (B) The microspore (pollen) of pine has one cell and two large air bladders that increase its buoyancy in air. The microspore develops into a small gametophyte in a process similar to that in pollen of flowering plants, except that a few more body cells are formed. First, two mitotic divisions produce two small body cells (C) that degenerate and a large cell that divides, resulting in a generative cell (D) and another body cell, called the tube cell (E). As in angiosperms, the body cell becomes the pollen tube, and the generative cell divides into two nonmotile sperm cells.
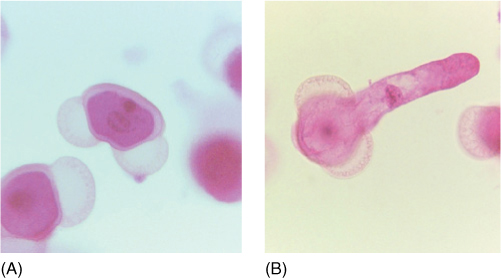
FIGURE 22-20 (A) The two air bladders of pine pollen are easily visible in this light micrograph. The addition of the hollow bladders adds virtually no weight to the pollen but increases its volume so that the pollen’s density (weight per volume) is decreased and it does not sink so quickly in air (× 200). (B) This pollen grain of pine has germinated and its tube cell is elongating (× 200).
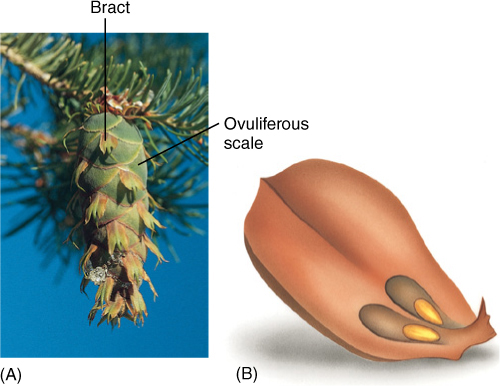
FIGURE 22-21 (A) The long three-pointed bracts of this Douglas fir (Pseudotsuga) cone are the true leaves of the cone axis. Each one contains an axillary bud whose leaves have fused together side by side into the flat, shield-like ovuliferous scale just behind each bract. These fused leaves that constitute the ovuliferous scale are actually megasporophylls. (B) If an ovuliferous scale were pulled from the cone and turned over, the two ovules would be visible. The scale obviously does not look like a set of leaves fused together, but look at Figure 22-6.
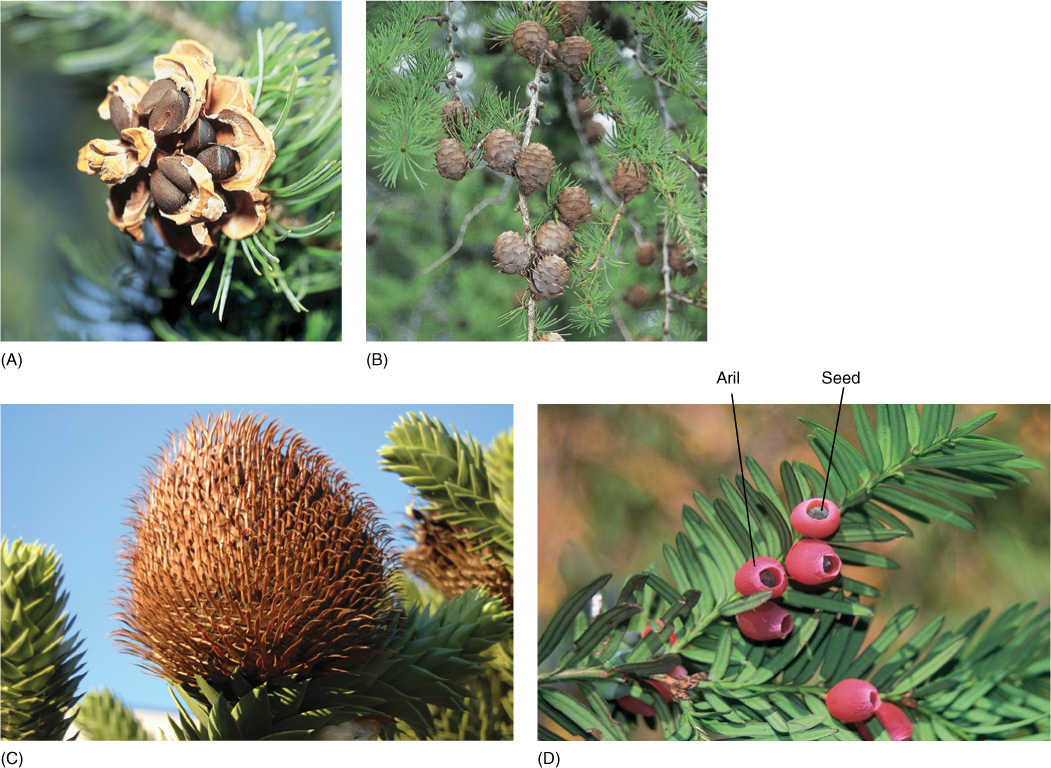
FIGURE 22-22 (A) In seed cones of pine, all parts are fused together; even the bract is fused to the ovuliferous scale. When morphologists first began working on pine cones without knowing about fossil structures, they were completely baffled and could not explain such a complex structure. (B) Larch (Larix species). (C) Monkey puzzle (Araucaria araucana). (D) Taxus has individual seeds, each surrounded by a red, fleshy aril (part of the seed coat); there are no ovulate cones in this genus.
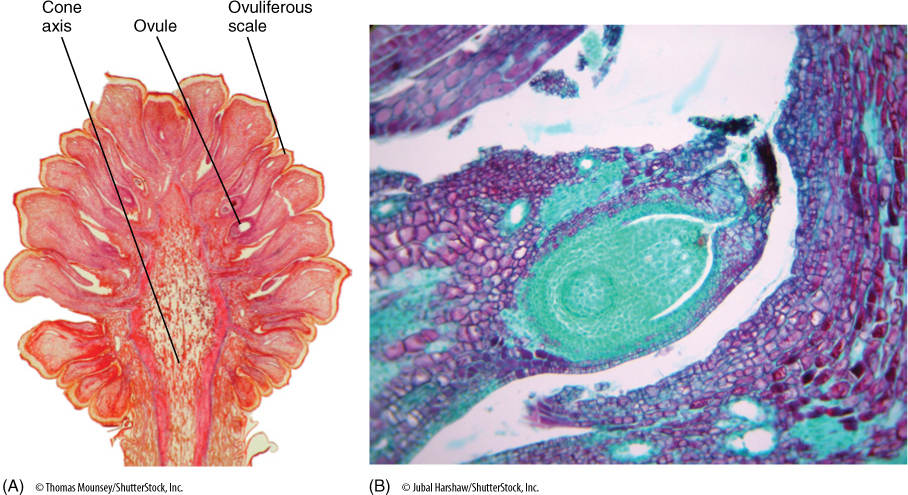
FIGURE 22-23 This section of a pine cone was made just as the megaspore mother cells were about to begin meiosis. Each ovuliferous scale carries two ovules, each of which contains an integument and a nucellus (the actual megasporangium). As in flowering plants, each nucellus usually has only one megasporocyte (megaspore mother cell) and produces only one surviving megaspore. (A) × 2. (B) × 25.
Conifer pollen arrives before the egg is mature, and more than a year may pass between pollination and fertilization. A massive pollen tube slowly digests its way toward the megagametophyte as the egg forms. Because the megasporangium does not open, a passageway digested by a pollen tube is necessary. This differs greatly from the free-living gametophytes of mosses and ferns, which have exposed archegonia that sperm swim into. The two or three eggs in one megagametophyte can all be fertilized, but only one zygote develops into an embryo; the other one or two die. A zygote does not immediately form an embryo in conifers; instead, some of the first cells elongate as a suspensor that pushes the other cells deep into the megagametophyte (FIGURE 22-25). These other cells, called the proembryo, develop into the embryo. There is no double fertilization as occurs in angiosperms; rather, the female gametophyte continues to grow and acts as a haploid nutritive tissue similar to endosperm. The mature embryo has the same organization as an angiosperm embryo (radicle, hypocotyl, epicotyl, and cotyledons) but always has many cotyledons, not just one or two. The seed also resembles that of a flowering plant, but it is borne in a cone, not a fruit. In two conifers, the cones become fleshy, fruit-like, and brightly colored—red in Podocarpus, blue in Juniperus.
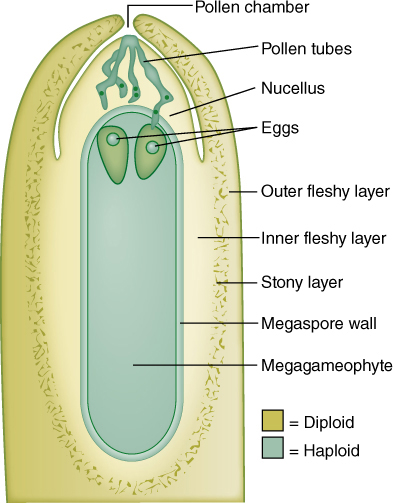
FIGURE 22-24 Ovules of pine and other conifers are much larger than those of flowering plants. This cellular megagametophyte is not as large as a moss or liverwort gametophyte, but it is much more plantlike than the megagametophytes of angiosperms. Above the two eggs is the thick, indehiscent megasporangium (nucellus). Sperms cannot swim to the archegonia and eggs; they must be carried by a pollen tube that digests its way through the megasporangium. Between the megasporangium and the integuments is the pollen chamber.
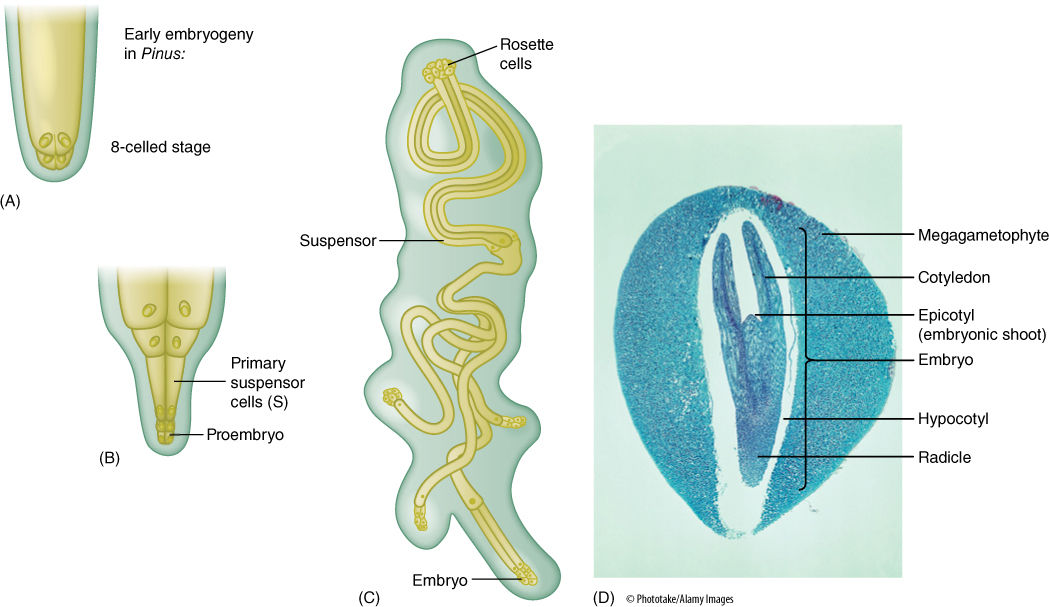
FIGURE 22-25 (A) Just as in angiosperms, the zygote of conifers produces a suspensor that thrusts the proembryo into the megagametophyte (B). (C) The suspensor can divide and form multiple embryos from each zygote, but only one embryo survives. (D) A conifer seed looks remarkably like the seed of a flowering plant. It has a seed coat (removed here) derived from the integument, an embryo with several cotyledons, and a nutritive tissue that is actually the megagametophyte, not endosperm. After fertilization, the parental sporophyte transports large amounts of nutrients, supplying everything needed for the embryos, which grow completely heterotrophically. It also fills all cells of the megagametophyte with carbohydrates, proteins, and mineral nutrients—this acts just like the endosperm of flowering plants and is often called endosperm (× 25).
![]() Division Cycadophyta: Cycads
Division Cycadophyta: Cycads
Modern cycads are frequently confused with either ferns or young palm trees because they have stout trunks with pinnately compound leaves (FIGURE 22-26). Most cycads are short plants less than 1 or 2 m tall, but Macrozamia can reach heights of 18 m. The trunk is covered with bark and persistent leaf bases that remain on the plant even after the lamina and petiole have abscised. Internally, cycad stems are similar to those of seed ferns—a thick cortex containing secretory ducts surrounds a small amount of manoxylic (parenchymatous) wood. Tracheids are long and wide, and rays are massive. Even very old stems have only a small amount of wood; most support is provided by the tough leaf bases. A prominent pith contains secretory canals.
Unlike seed ferns, cycad foliage leaves do not bear ovules. Instead, cycads produce seed cones and pollen cones, each on separate plants; cycads are always dioecious. Pollen cones consist of spirally arranged shield-shaped microsporophylls that bear clusters of microsporangia (FIGURE 22-27). Upon germination, pollen grains produce a branched pollen tube and large, multiflagellated sperm cells. Seed cones are variable, with those of Cycas revoluta usually considered the most relictual (FIGURE 22-28). In this species, the seed cone is a large, loose aggregation of leaf-like, pinnately compound megasporophylls. Six to eight large ovules occur near the base, but the upper half of the megasporophyll is rather leaf like, similar to the ovule-bearing organs of some seed ferns. Megasporophylls of the cycad Zamia floridana are considered more derived. They are shield-shaped and bear only two ovules, and the entire cone is quite compact. Cycad ovules are like those of seed ferns, having a large, vascularized megasporangium and a loosely attached, vascularized integument.
Although Cycadophyta was a much larger group with many more species in earlier times, currently, it contains nine or ten genera and approximately 100 species. Modern cycads are highly prized ornamentals in the warmest parts of the United States; only a few can withstand freezing temperatures in winter. They are almost all tropical with an unusual distribution: Some occur in Cuba and Mexico, others in Australia, still others in southeast Asia or Africa. Cycads are now believed to have been widespread and to have occupied many habitats, but in most areas, they have become extinct. The few remaining, widely scattered species are the survivors of a formerly extensive group.
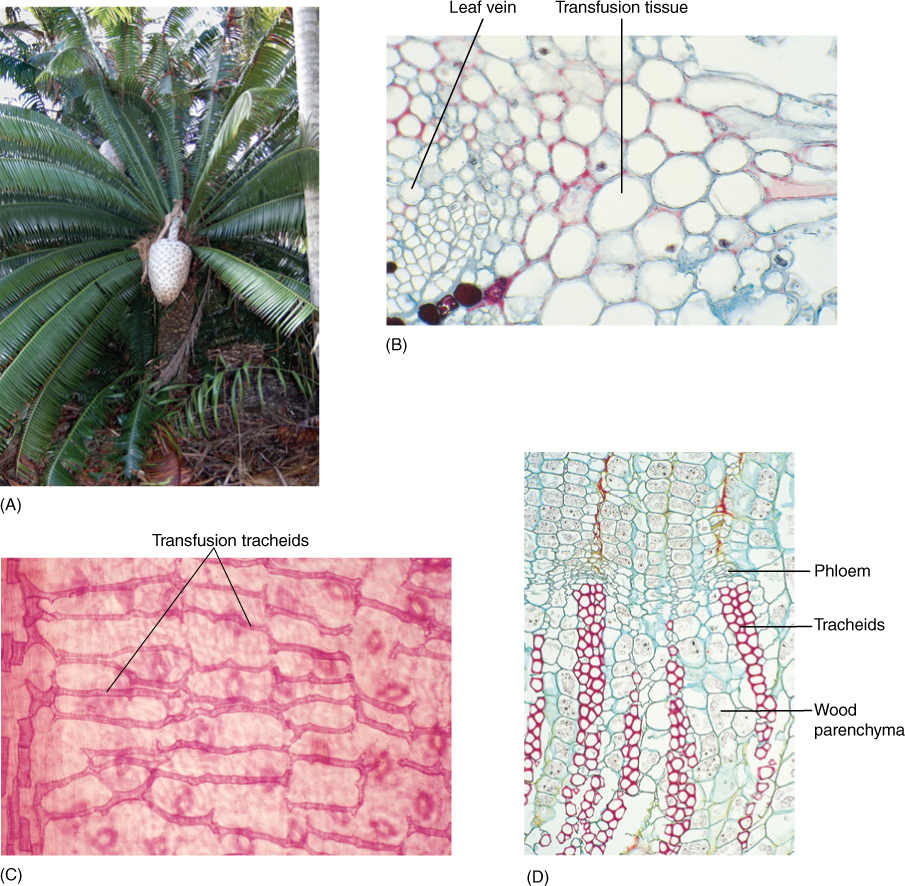
FIGURE 22-26 Aspects of the vegetative body of cycads. (A) Dioon has a short, stout trunk with tough, leathery, pinnately compound leaves; note the huge seed cone. (B) Leaf veins with transfusion tissue similar to that in conifer leaves (× 50). (C) Transfusion tracheids extend from veins into the leaf blade. Stomata are visible, slightly out of focus, in the lower epidermis (× 32). (D) Cycads have only small amounts of wood; it is parenchymatous (× 32).
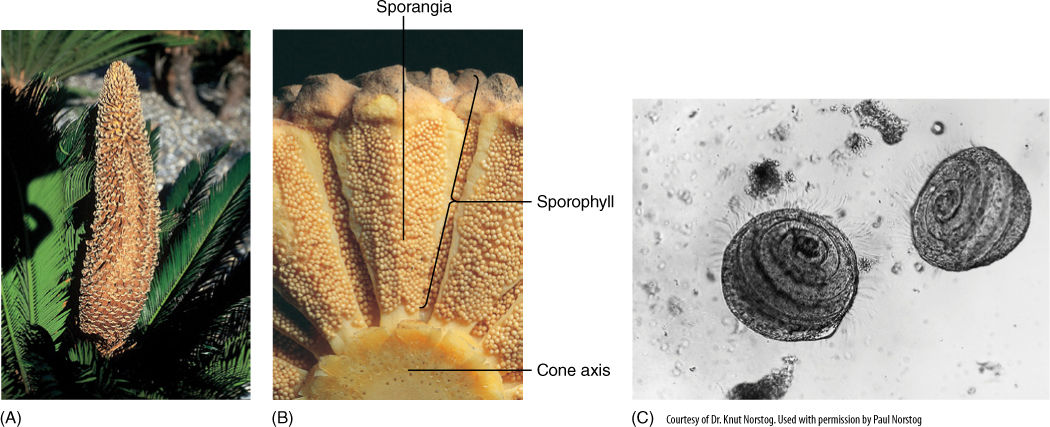
FIGURE 22-27 (A) Pollen cone, more than 30 cm long, in Cycas circinnalis. It is a simple cone, with one axis that bears microsporophylls. (B) Microsporophylls in cycads may bear many microsporangia. (C) Sperm cells of cycads have hundreds of flagella and must swim to the egg cell. Zamia (× 165).
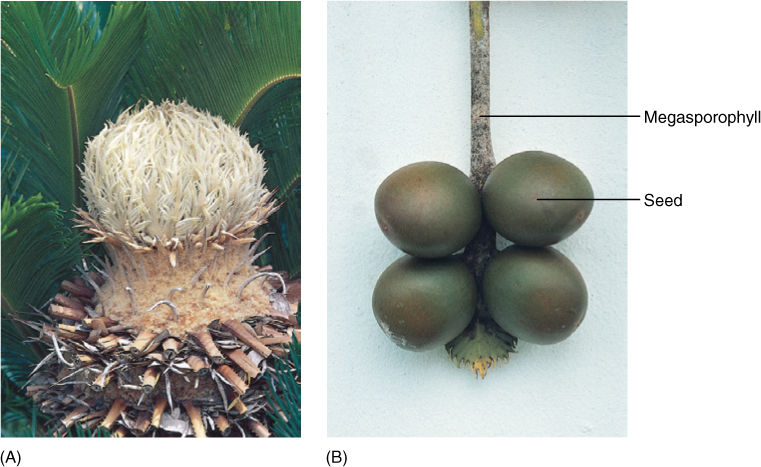
FIGURE 22-28 (A) Cycad seed cones are simple, with megasporophylls borne on the only axis. They have no bracts or axillary buds. (B) Cycad megasporophylls are not fused structures as are ovuliferous scales of conifers; instead, the megasporophyll is simple and strongly resembles a foliage leaf, at least in Cycas circinnalis. It is not difficult to see how this type of sporophyll could evolve from a seed fern sporophyll (see Figure 22-11).
![]() Division Cycadeoidophyta: Cycadeoids
Division Cycadeoidophyta: Cycadeoids
The cycadeoids (all extinct) had vegetative features almost identical to those of cycads (FIGURE 22-29). The two groups differ only in subtle details of the differentiation of stomatal complexes and in leaf trace organization. On such characters alone, cycadeoids would never be considered distinct from cycads; however, individual cones of cycadeoids contained both microsporophylls and megasporophylls. Each ovule had a stalk, and the megasporangium was surrounded by an integument that extended out into a long micropyle. Between the ovules were thick, fleshy scales. Microsporophylls were located below the cluster of megasporophylls and curved upward, enveloping the megasporophylls. Each microsporophyll was cup shaped and contained numerous microsporangia.
![]() Division Ginkgophyta: Maidenhair Tree
Division Ginkgophyta: Maidenhair Tree
This division contains a single living species, Ginkgo biloba (FIGURE 22-30). It may seem unusual to erect an entire division for a single species, but G. biloba (the “maidenhair tree”) is itself unusual. It looks very much like a large dicot tree with a stout trunk and many branches, but its wood is like that of conifers: It lacks vessels and axial parenchyma. It has “broad leaves,” but they have dichotomously branched veins like seed ferns, not reticulate venation like dicots. Ginkgos have both short shoots, which bear most of the leaves, and long shoots. Reproduction in Ginkgo is dioecious and gymnospermous, but cones are not produced. Instead, ovules occur in pairs at the ends of a short stalk and are completely unprotected at maturity. Pollen is produced in an organ that resembles a catkin, having a stalk and several sporangiophores that each have two microsporangia. Like ovules of pteridosperms and cycads, the ovules of Ginkgo are large (1.5 to 2.0 m in diameter) and develop a three-layered seed coat.

FIGURE 22-29 Vegetatively, cycadeoids such as Cycadeoidea were similar to cycads. They had a broad cortex and a tough outer protective layer formed by persistent leaf bases.

FIGURE 22-30 (A) A tree of Ginkgo biloba could easily be mistaken for a dicot tree. (B) Ginkgo leaves are broad. They have dichotomous venation like leaves of cycads and seed ferns, not reticulate venation like dicots. (C) Microsporophylls occur in small, cone-like clusters (think of pine pollen cones [Figure 22-18], not pine seed cones [Figure 22-22]) mixed with foliage leaves (petioles are visible) on short shoots. (D) Ovules occur in pairs at the end of a stalk-like megasporophyll. The ovule itself, the integument, is exposed; no bract or ovuliferous scale protects it.
A Ginkgo tree itself is beautiful and is a popular ornamental because the leaves turn brilliant yellow in autumn, but the microsporangiate (“male”) trees are preferred; when the megasporangiate (“female”) trees produce seeds, the outer fleshy layer of the seed emits butyric acid, which has a putrid odor that is difficult to tolerate.
The exact ancestors of ginkgos are not known, but they must have been one of the seed ferns or a closely related group. The cladogram of Figure 22-2 proposes that cycads and ginkgos have a common ancestor, but DNA analysis cannot include the seed ferns, all of which are extinct. Ginkgos became abundant during the Mesozoic Era, especially in the mid Jurassic Period (approximately 170 million years ago). Fossilized remains of leaves and wood can be found in almost all areas of the world, especially in high latitudes such as Alaska, Canada, and Siberia near the North Pole, and Patagonia, South Africa, and New Zealand near the South Pole. Ginkgos began to die out early in the Tertiary Period, but two species, G. biloba and G. adiantoides, persisted. The latter species became extinct during the Pliocene Epoch, approximately 10 to 12 million years ago.
![]() Division Gnetophyta
Division Gnetophyta
Gnetophyta (pronounced as if spelled neat AH fit a) contains three groups of enigmatic plants: Gnetum with 30 species (FIGURE 22-31), Ephedra with about 40 species (FIGURE 22-32), and Welwitschia mirabilis, the only species in the genus (FIGURE 22-33).
Gnetums are mostly vines or small shrubs with broad leaves similar to those of dicots. They are native to southeast Asia, tropical Africa, and the Amazon Basin. Plants of Ephedra are tough shrubs and bushes that are very common in desert regions in northern Mexico and southwestern United States and dry mountains in South America. Their leaves are reduced and scale like. The few living plants of Welwitschia exist only in deserts of South Africa or in cultivation. They have a short, wide stem and only two leaves, but the leaves grow perennially from a basal meristem, becoming increasingly longer.

FIGURE 22-31 Gnetum. (A) Plants of Gnetum strongly resemble dicots, having broad leaves and woody stems. (B) Ovules are not borne in cones.

FIGURE 22-32 Ephedra. (A) Plants of Ephedra often occur in dry areas and strongly resemble many types of desert-adapted dicots. Although their reproductive structures are gymnospermous, the microsporangiate cone (B) could be mistaken for a staminate imperfect flower. The naked ovules reveal that they are not angiosperms (C).
All three genera are unusual in being gymnosperms with vessels in their wood. This had been thought to show that they might be related to primitive angiosperms; however, their vessel elements evolved from tracheids with circular bordered pits, whereas those of angiosperms were derived from scalariform tracheids. Furthermore, angiosperms are thought to have evolved from vessel-less ancestors, the vessels evolving after flowers, not before them.
Unlike the pollen cones of all other gymnosperms, those of gnetophytes are compound and contain small bracts. Seed cones are also compound and contain extra layers of tissue around the ovules; the tissue is variously interpreted as an extra integument, bract, or sporophyll.
The few fossils of gnetophyte organs or tissues are only several million years old, too recent to be of much help in understanding the evolution and ancestry of the group. The pollen is distinctive, being spindle-shaped and having narrow ridges. It is easy to recognize, and fossil pollen of this type occurs as far back as the late Triassic Period, but pollen has not helped reveal their origins either. Figure 22-2 includes them as one of several groups emerging from the same point. Certain aspects of their anatomy and reproduction have been interpreted as indicating that gnetophytes and flowering plants constitute two sister clades with a common ancestor. If so, the two would form the group “Division Anthophyta” or “anthophytes”; however, there are too few data to be confident of this, and currently, it is best to recall the principle of skepticism, realize that we do not yet know, and must continue searching for more data about their phylogenetic relationships. Remember that Figure 22-2 is a model, a hypothesis.

FIGURE 22-33 Welwitschia mirabilis. (A) Whole plant with torn leaves. (B) Microsporangiate strobili. (C) Megasporangiate strobilus.
 At the Next Level
At the Next Level
1. Animals and gymnosperms. Cooperation between animals and angiosperms is widespread: Animals pollinate flowers and disperse fruits. This also occurs with gymnosperms more often than is commonly realized. Some birds cache seeds of conifers in several locations, and then forget about some of them, which are thus able to germinate far from the parent tree. The bright red arils of Taxus and the fleshy soft blue cones of junipers attract animals. The flesh-covered seeds of maidenhair tree (Ginkgo) must have been eaten by some animal that is now extinct. Cycads have such large pollen cones that wind is probably the main pollination vector, but beetles have been found carrying pollen from plant to plant. You may have to gather information bit by bit, but the interactions of animals and gymnosperms is an interesting, underappreciated area.
2. Conifers in the last 100 million years. Giant redwood trees now exist in just a few valleys in California and need all the protection we can give them so they do not go extinct. But several million years ago they were abundant and widespread in extensive forests. Starting about 50 million years ago, Earth became cooler and its climate changed (fortunately, humans did not exist yet, so we definitely did not cause that episode of climate change). The redwoods provide a good example of how some species react as conditions change, how refugia are important to the survival of species. The same thing happened to many other species and genera of conifers in the last 100 million years. The ecology of redwoods is well-known and worth examining as an introduction to the paleoecology of conifers in general.
3. Cycad sperm cells swim with hundreds of flagella. We don’t usually think of plant cells as being good swimmers, but in fact the sperm cells of all cryptogams (plants without seeds) and several species of seed plants must swim to the egg cell. In fact the sperm cells of cycads put those of animals to shame: Cycad sperm cells are large and have hundreds of flagella arranged in a long spiral that wraps around half the cell. Just as in animal flagella, plant flagella need a basal body, and these must be produced by the hundreds as each cycad sperm cell matures. The cell biology of cycad sperms is a bit different from most plant cell biology, but is definitely interesting.
SUMMARY
1. In a seed, the embryo draws its nutrition from the surrounding megagametophyte (or endosperm), which in turn is supplied with nutrients by the parental sporophyte. The sporangium and associated structures provide protection for the megaspore, megagametophyte, and embryo.
2. In all modern seed plants, the megasporangium (nucellus) does not open; therefore, a pollen tube must deliver the sperm cells to the archegonia. Sperm cells are still motile in many seed plants.
3. Progymnosperms arose from trimerophytes with the evolution of a vascular cambium of unlimited growth potential which produced solid wood with little parenchyma. The evolution of true seeds also began in the progymnosperms, first with retention of the megaspore and then with formation of an integument.
4. One order of progymnosperms, Archaeopteridales, gave rise to conifers; another progymnosperm order, Aneurophytales, evolved into pteridosperms, cycads, cycadeoids, and perhaps angiosperms.
5. Conifers constitute a diverse division of large trees with solid wood, simple pollen cones, and compound seed cones.
6. Pteridosperms (seed ferns) gave rise to cycads and cycadeoids. Important evolutionary changes were formation of pith and of ovules on more or less unspecialized foliage leaves. Their wood contained abundant axial parenchyma.
7. Cycads are modern seed plants with compound leaves, parenchymatous wood, and simple seed cones consisting of an axis and a rather leaflike megasporophyll.
8. Ginkgo biloba is the sole living species of its division. It resembles a dicot tree except that its leaves have dichotomous veins and it bears naked ovules, surrounded by neither a cone nor a carpel.
9. Reproduction in gnetophytes is gymnospermous, but plants of Ephedra and Gnetum resemble dicots. Welwitschia has only two leaves, which grow indeterminately from basal meristems.
IMPORTANT TERMS
angiosperms
anthophytes
compound cones
cone bracts
gymnosperms
integument
lignophytes
long shoots
manoxylic wood
micropyle
ovuliferous scale
pollen chamber
proembryo
progymnosperms
pycnoxylic wood
seed ferns
short shoots
simple cones
spermatophytes
suspensor
REVIEW QUESTIONS
1. What is the disadvantage of a life cycle in which alternate generations are completely independent of the preceding generation? Are there advantages to this type of life cycle?
2. What is a cone? How are cones of conifers similar to those of lycophytes and cycads? How do they differ?
3. The classification of the seed plants has varied. If a person groups all seed plants together in one large Division Spermatophyta, then they have two classes—the class ________________ with naked seeds and the class __________________, plants with carpels. On the other hand, many people (and this book) use four divisions, not one. List the three divisions of “gymnosperms” and the one division of flowering plants.
4. If angiosperms evolved from some type of gymnosperm, then the group “gymnosperms” is not natural. Why not? In cladistic terms, an incomplete group is a __________________group.
5. Describe progymnosperms. What were the significant evolutionary advances that characterized progymnosperms?
6. The section Evolution of Seeds describes the fossil seeds Archaeosperma arnoldii. Its megasporangium was surrounded by a layer of tissue, called an _________________ that projected upward, and there was a large _______________, a hole that permitted sperm cells to swim to the egg. If you have studied flowering plant reproduction, how many of these same features occur in flowering plants?
7. In progymnosperms, microgametophytes produced and released sperm cells that could swim—but not very far. What is a pollen chamber and how did it help fertilization?
8. Are any conifers herbs? Annuals? Vines? Do all conifers have needles like pines (Hint: Figure 22-13).
9. Describe a conifer pollen cone. Why is it a simple cone? Describe a conifer seed cone. Why is it a compound cone?
10. Seed cones of conifers are more complex than pollen cones. Seed cones are ________________ cones, each consisting of a shoot with axillary buds. The main axis of the cone bears leaves called ________________, rather than sporophylls, and each of these has an axillary bud that bears the ___________________. The axillary bud is microscopic, and its _________________ are fused laterally, forming an ____________________ scale.
11. Unlike pollination in flowering plants, conifer pollen arrives before the egg is mature, and more than a ___________________ may pass between pollination and fertilization. The pollen germinates, and a massive ____________________ slowly digests its way toward the ___________________ as the egg forms.
12. In most conifers, as the seeds mature, the cone scales and ovuliferous scales become hard and tough—a typical pine cone (although firs have fir cones, not pine cones, and larches have larch cones, and so on); however, in ________________, the cone becomes red and fleshy, and in ________________, it becomes blue and fleshy.
13. Seed ferns are a group of extinct plants; they resembled tree ferns, but what did they have on their leaves instead of sori?
14. There is a good chance you have seen cycads. What do they look like? What two other types of plants can they be confused with (Hint: Some are called “cardboard palms”)? Do cycads ever have simple leaves?
15. Do any seed plants have flagella? Do their sperm cells have flagella? Look at Figure 22-27.
16. What is the one and only species in the Division Ginkgophyta? Being part of the “gymnosperms,” does it have needle-shaped or scale-shaped leaves? The leaf venation is unusual because the leaves have dichotomously branched veins like ___________________, not reticulate venation like ________________________.
17. What are the three groups classified in Gnetophyta?
18. Would it have been possible, on a theoretical basis, for an indehiscent megasporangium to evolve before integument-penetrating pollen tubes evolved? Why or why not? What problem would be involved?
Design Credits: Hummingbird: © Tongho58/Moment/Getty; Green Plant Cells: © ShutterStock, Inc./Nataliya Hora; Purple Tulip: © ShutterStock, Inc./Marie C Fields; Dandelion: © ShutterStock, Inc./Danielkreissl; Poppy: © ShutterStock, Inc./Saruri; Plant Icon: © ShutterStock, Inc./Vector; Digging Man Icon: © ShutterStock, Inc./Z-Art