CONCEPTS IN BIOLOGY
PART II. CORNERSTONES: CHEMISTRY, CELLS, AND METABOLISM
3. Organic Molecules—The Molecules of Life

Down the Toilet—But Then What?
Scientists Increasingly Concerned About the Water We Drink.
It has been reported that a vast array of pharmaceuticals have been found in the drinking water supplies of at least 41 million Americans. Many of these are organic compounds and include antibiotics, anti-convulsants, mood stabilizers, and sex hormones. Organic compounds can be very complex and long-lasting molecules. How do these drugs get into the water? One way is by unmetabolized drugs that are excreted in urine. Also, healthcare providers recommend that unused medications be flushed down the toilet. In addition, these compounds get into the water supply on occasion by accidental spills that can result in contamination of our water. One U.S. Environmental Protection Agency (EPA) administrator stated that this problem is a growing concern. The EPA is taking this issue seriously because neither sewage treatment nor water purification can remove all drugs.
As scientists learn from their research, more specific questions are formulated that relate to long-term health effects of pharmaceutical contamination of our water. For example, a popular osteoporosis drug, Fosamax, has been linked to severe musculoskeletal pain and a serious bone disease called osteonecrosis of the jaw (ONJ), also known as “dead jaw” and “fossy jaw.” Microbial biofilms, a mix of bacteria and sticky organic compounds, appear to be the cause of this side effect. If the drugs reach high enough levels of contamination in our water supply, will we see an increase in ONJ in people who don’t even take this medication?
Some dentists are observing the eruption of second molar teeth in children as young as 8 years old. Normally these do not appear until a person is about 12 or 13 years old. Might there be a cause-and-effect relationship with some pharmaceutical contaminant in our water supply?
• What makes organic molecules different from other molecules?
• What is the structure of various organic compounds?
• Is there a point at which the cure is worse than the disease?
ü Background Check
Concepts you should already know to get the most out of this chapter:
• The nature of matter (chapter 2)
• Chemical changes that can occur in matter (chapter 2)
• The key characteristics of water (chapter 2)
• The different types of chemical reactions (chapter 2)
3.1. Molecules Containing Carbon
The principles and concepts discussed in chapter 2 apply to all types of matter—nonliving as well as living. Living systems are composed of various types of molecules. Most of the chemicals described in chapter 2 do not contain carbon atoms and, so, are classified as inorganic molecules. This chapter is mainly concerned with more complex structures, organic molecules, which contain carbon atoms arranged in rings or chains. The words organic, organism, organ, and organize are all related. Organized objects have parts that fit together in a meaningful way. Organisms are living things that are organized. Animals, for example, have organ systems within their bodies, and their organs are composed of unique kinds of molecules that are organic.
The original meanings of the terms inorganic and organic came from the fact that organic materials were thought either to be alive or to be produced only by living things. A very strong link exists between organic chemistry and the chemistry of living things, which is called biochemistry or biological chemistry. Modern chemistry has considerably altered the original meanings of the terms organic and inorganic, because it is now possible to manufacture unique organic molecules that cannot be produced by living things. Many of the materials we use daily are the result of the organic chemist’s art. Nylon, aspirin, polyurethane varnish, silicones, Plexiglas, food wrap, Teflon, and insecticides are just a few of the unique synthetic molecules that have been invented by organic chemists (figure 3.1). Plastics such as low-density polyethylene (LDPE) used to make garbage bags are extremely stable molecules that require hundreds of years to break down.

FIGURE 3.1. Some Common Synthetic Organic Materials
These are only a few examples of products containing useful organic compounds invented and manufactured by chemists.
Many organic chemists have taken their lead from living organisms and have been able to produce organic molecules more efficiently, or in forms that are slightly different from the original natural molecules. Some examples of these are rubber, penicillin, certain vitamins, insulin, and alcohol (figure 3.2). Another example is the insecticide Pyrethrin. It is based on a natural insecticide and is widely used for agricultural and domestic purposes. It is derived from a certain type of chrysanthemum plant, Pyrethrum cinerariaefolium.

FIGURE 3.2. Natural and Synthetic Organic Compounds
(a) This researcher is testing antibiotics produced by microbes isolated from the environment. Each paper disk, containing a different antibiotic, is placed on the surface of a Petri dish with growing, disease- causing bacteria. The presence of a “dead zone” around a disk indicates that the antibiotic has spread through the gel and is able to inhibit or kill the bacteria. (b) Certain types of chrysanthemums produce the insecticide Pyrethrin. (c) It can be found as an “active ingredient” in many commercially available ant- and cockroach-killing products.
Carbon: The Central Atom
All organic molecules, whether natural or synthetic, have certain common characteristics. The carbon atom, which is the central atom in all organic molecules, has some unusual properties that contribute to the nature of an organic compound. Carbon is unique in that it can combine with other carbon atoms to form long chains. In many cases, the ends of these chains may join together to form rings (figure 3.3).
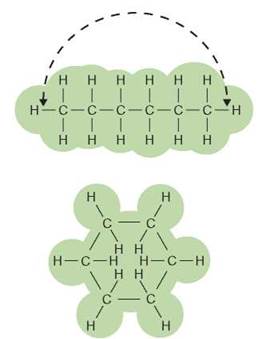
FIGURE 3.3. Chain and Ring Structures
The ring structure shown on the bottom is formed by joining the two ends of a chain of carbon atoms.
Also unusual is that these bonding sites are all located at equal distances from one another because the 4 outer-most electrons do not stay in the standard positions described in chapter 2. They distribute themselves differently, enabling them to be as far apart as possible (figure 3.4). Carbon atoms are usually involved in covalent bonds. Because carbon has four places it can bond, the carbon atom can combine with four other atoms by forming four separate, single covalent bonds with other atoms. This is the case with the methane molecule, which has four hydrogen atoms attached to a single carbon atom (review figure 3.4). Pure methane is a colorless, odorless gas that makes up 95% of natural gas. The aroma of natural gas is the result of mercaptan and trimethyl disulfide added for safety to let people know when a leak occurs.
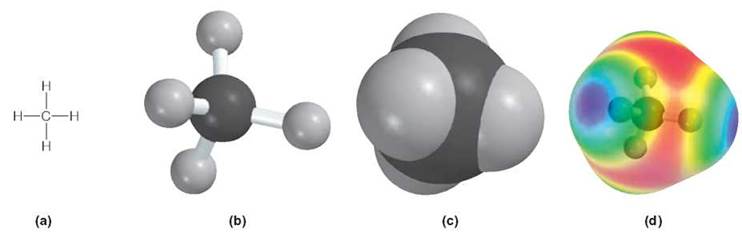
FIGURE 3.4. Models of a Methane Molecule
The structures of molecules can be modeled in many ways. For the sake of simplicity, diagrams of molecules such as the gas methane can be (a) two-dimensional drawings, although in reality they are three-dimensional molecules and take up space. The model shown in (b) is called a ball-and-stick model. Part (c) is a space-filling model, while (d) is a computer-generated model. Each time you see the various ways in which molecules are displayed, try to imagine how much space they actually occupy.
Some atoms may be bonded to a single atom more than once. This results in a slightly different arrangement of bonds around the carbon atom. An example of this type of bonding occurs when oxygen is attracted to a carbon. An atom of oxygen has 2 electrons in its outermost energy level. If it shares 1 of these with a carbon and then shares the other with the same carbon, it forms a double covalent bond. A double bond is two covalent bonds formed between two atoms that share two pairs of electrons. Oxygen is not the only atom that can form double bonds, but double bonds are common between oxygen and carbon. The double bond is denoted by two lines between the two atoms:
![]()
Since carbon has 4 electrons in its outer energy level, two carbon atoms might form double bonds between each other and then bond to other atoms at the remaining bonding sites. Figure 3.5 shows several compounds that contain double bonds.
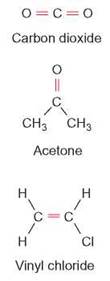
FIGURE 3.5. Double Bonds
These diagrams show several molecules that contain double bonds in red. A double bond is formed when two atoms share two pairs of electrons with each other.
Some organic molecules contain triple covalent bonds; the flammable gas acetylene, HC=CH, is one example. Others—such as hydrogen cyanide HC≡N—have biological significance. This molecule inhibits the production of energy and can cause death as can other organic molecules. (How Science Works 3.1).
HOW SCIENCE WORKS 3.1
Organic Compounds: Poisons to Your Pets!
The opening vignette concerning the pharmaceutical contamination of our water supply has far-reaching health implications to humans. However, we should not forget our pets, whose metabolism is not necessarily the same as that of humans. Organic compounds can affect them differently—most people have organic compounds around the house or garage that are toxic to dogs.
Ibuprofen—This nonsteroidal, anti-inflammatory (NSAID) might help relieve the pain of a person's headache, but if ingested by a dog, it can cause stomach and kidney problems in the animal. It can also alter the dog's nervous system, resulting in depression and seizures.

Acetaminophen—While a common pain medication for people, this drug can cause liver failure, swelling of the face and paws, and a problem with oxygen transport in the blood in a dog. If a dog ingests acetaminophen, it will probably need to be hospitalized.
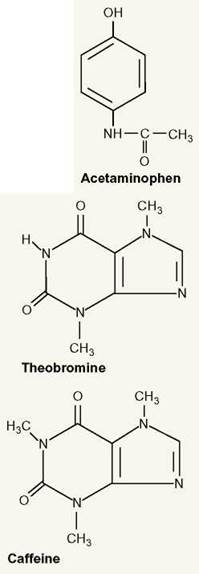
Chocolate—Two toxic compounds in chocolate are theobromine and caffeine. Theobromine is found in candy, tea, and cola beverages. Since dogs and kittens metabolize this compound very slowly, it can remain in their bodies long enough to cause nausea, vomiting, diarrhea, and increased urination. Depending on the amount of chocolate the pet has ingested, it can also cause seizures, internal bleeding, heart attacks, and eventually death. Caffeine in coffee, tea, and cola drinks can result in vomiting, diarrhea, tremors, heart arrhythmias, and seizures in pets. Notice how similar the molecular structure of theobromine is to caffeine.
The Complexity of Organic Molecules
Although many kinds of atoms can be part of an organic molecule, only a few are commonly found. Hydrogen (H) and oxygen (O) are almost always present. Nitrogen (N), sulfur (S), and phosphorus (P) are also very important in specific types of organic molecules.
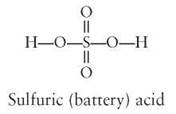
An enormous variety of organic molecules is possible, because carbon is able to (1) bond at four different places, (2) form long chains and rings, and (3) combine with many other kinds of atoms. The types of atoms in the molecule are important in determining the properties of the molecule. The three-dimensional arrangement of the atoms within the molecule is also important. Because most inorganic molecules are small and involve few atoms, a group of atoms can be usually arranged in only one way to form a molecule. There is only one arrangement for a single oxygen atom and two hydrogen atoms in a molecule of water. In a molecule of sulfuric acid, there is only one arrangement for the sulfur atom, the two hydrogen atoms, and the four oxygen atoms.
However, consider these two organic molecules:
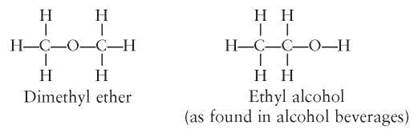
Both the dimethyl ether and the ethyl alcohol contain two carbon atoms, six hydrogen atoms, and one oxygen atom, but they are quite different in their arrangement of atoms and in the chemical properties of the molecules. The first is an ether; the second is an alcohol. Because the ether and the alcohol have the same number and kinds of atoms, they are said to have the same empirical formula, which in this case can be written C2H6O. An empirical formula simply indicates the number of each kind of atom within the molecule. The arrangement of the atoms and their bonding within the molecule are indicated in a structural formula. Figure 3.6 shows several structural formulas for the empirical formula C6H12O6. Molecules that have the same empirical formula but different structural formulas are called isomers.
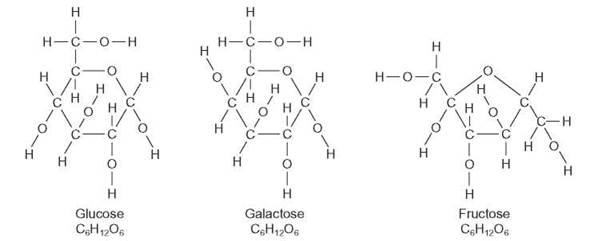
FIGURE 3.6. Structural Formulas for Several Hexoses
Three 6-carbon sugars—hexoses (hex = 6; -ose = sugar)—are represented here. All have the same empirical formula (C6H12O6), but each has a different structural formula. These three are called structural isomers. Structural isomers have different chemical properties from one another.
The Carbon Skeleton and Functional Groups
At the core of all organic molecules is a carbon skeleton, which is composed of rings or chains (sometimes branched) of carbon. It is this carbon skeleton that determines the overall shape of the molecule. The differences among various kinds of organic molecules are determined by three factors: (1) the length and arrangement of the carbon skeleton, (2) the kinds and location of the atoms attached to it, and (3) the way these attached atoms are combined. These specific combinations of atoms, called functional groups, are frequently found on organic molecules. The kind of functional groups attached to a carbon skeleton determine the specific chemical properties of that molecule. By learning to recognize some of the functional groups, you can identify an organic molecule and predict something about its activity. Figure 3.7 shows some of the functional groups that are important in biological activity. Remember that a functional group does not exist by itself; it is part of an organic molecule. Outlooks 3.1 explains how chemists and biologists diagram the kinds of bonds formed in organic molecules.
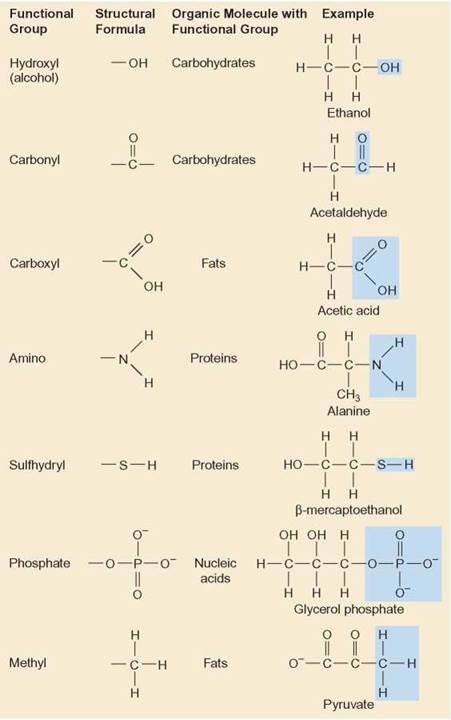
FIGURE 3.7. Functional Groups
These are some of the groups of atoms that frequently attach to a carbon skeleton. The nature of the organic compound changes as the nature of the functional group changes from one molecule to another.
Macromolecules of Life
Macromolecules (macro = large) are very large organic molecules. We will look at four important kinds of macromolecules: carbohydrates, proteins, nucleic acids, and lipids. Carbohydrates, proteins, and nucleic acids are all polymers (poly = many; mer = segments). Polymers are combinations of many smaller, similar building blocks called monomers (mono = single) bonded together (figure 3.8).
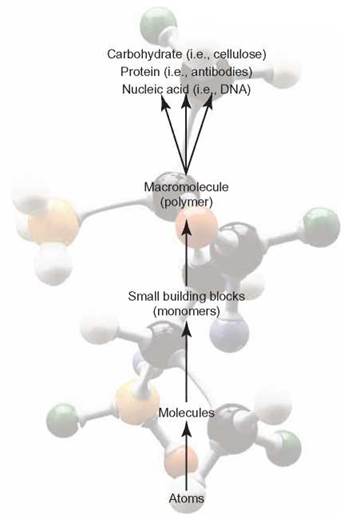
FIGURE 3.8. Levels of Chemical Organization
As a result of bonding specific units of matter in specific ways, molecules of enormous size and complexity are created.
Although lipids are macromolecules, they are not polymers. A polymer is similar to a pearl necklace or a boat’s anchor chain. All polymers are constructed of similar segments (pearls or links) hooked together to form one large product (necklace or anchor chain).
The monomers in a polymer are usually combined by a dehydration synthesis reaction (de = remove; hydro = water; synthesis = combine). This reaction occurs when two smaller molecules come close enough to have an —OH removed from one and an —H removed from the other. These are combined to form a new water molecule (H2O), and the remaining two segments are combined to form the macromolecule.
Figure 3.9a shows the removal of water from between two monomers. Notice that, in this case, the structural formulas are used to help identify where this is occurring. The chemical equation also indicates the removal of water. You can easily recognize a dehydration synthesis reaction, because the reactant side of the equation shows numerous, small molecules, whereas the product side lists fewer, larger products and water.
The reverse of a dehydration synthesis reaction is known as hydrolysis (hydro = water; lyse = to split or break). Hydrolysis is the process of splitting a larger organic molecule into two or more component parts by adding water (figure 3.9b). The digestion of food molecules in the stomach is an example of hydrolysis.
OUTLOOKS 3.1
Chemical Shorthand
You have probably noticed that sketching the entire structural formula of a large organic molecule takes a great deal of time. If you know the structure of the major functional groups, you can use several shortcuts to more quickly describe chemical structures. When multiple carbons with 2 hydrogens are bonded to each other in a chain, it is sometimes written as follows:
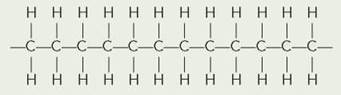
It can also be written:
![]()
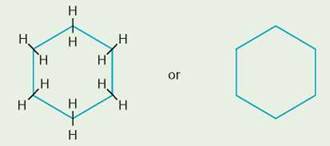
More simply, it can be written(—CH2—)12. If the 12 carbons were in a pair of two rings, we probably would not label the carbons or hydrogens unless we wished to focus on a particular group or point. We would probably draw the two 6-carbon rings with only hydrogen attached as follows:
![]()
Don't let these shortcuts throw you. You will soon find that you will be putting a —H group onto a carbon skeleton and neglecting to show the bond between the oxygen and hydrogen. Structural formulas are regularly included in the package insert information of most medications.

3.1. CONCEPT REVIEW
1. What is the difference between inorganic and organic molecules?
2. What two characteristics of the carbon molecule make it unique?
3. Diagram an example of the following functional groups: amino, alcohol, carboxyl.
4. Describe five functional groups.
5. List three monomers and the polymers that can be constructed from them.