CONCEPTS IN BIOLOGY
PART II. CORNERSTONES: CHEMISTRY, CELLS, AND METABOLISM
6. Biochemical Pathways—Cellular Respiration
6.2. An Overview of Aerobic Cellular Respiration
Aerobic cellular respiration is a specific series of enzyme- controlled chemical reactions in which oxygen is involved in the breakdown of glucose into carbon dioxide and water; the chemical-bond energy from glucose is released to the cell in the form of ATP. The following equation summarizes this process as it occurs in your cells and those of many other organisms:
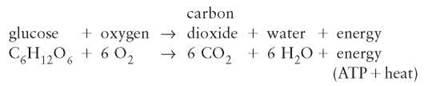
Covalent bonds are formed by atoms sharing pairs of fast-moving, energetic electrons. Therefore, the covalent bonds in the sugar glucose contain chemical potential energy. The removal of the electrons from glucose results in glucose being oxidized. Of all the covalent bonds in glucose (O—H, C—H, C—C), those easiest to get at are the C—H and O—H bonds on the outside of the molecule. When these bonds are broken, two things happen:
1. The energy of the electrons can ultimately be used to phosphorylate ADP molecules to produce higher-energy ATP molecules.
2. Hydrogen ions (protons) are released and pumped across membranes, creating a gradient. When they flow back to the side from which they were pumped, their energy is used to generate even more ATP (refer to chapter 5, Proton Pump).
These high-energy electrons cannot be allowed to fly about at random because they would quickly combine with other molecules, causing cell death. Electron-transfer molecules, such as NAD+ and FAD, hold electrons temporarily before passing them on to other molecules. ATP is formed when these transfers take place (see chapter 5). Once energy has been removed from electrons for ATP production, the electrons must be placed in a safe location. In aerobic cellular respiration, these electrons are ultimately attached to oxygen. Oxygen serves as the final resting place of the less energetic electrons. When the electrons are added to oxygen, it becomes a negatively charged ion, O=.
Because the oxygen has gained electrons, it has been reduced. Thus, in the aerobic cellular respiration of glucose, glucose is oxidized and oxygen is reduced. A molecule cannot simply lose its electrons—they have to go someplace! If something is oxidized (loses electrons), something else must be reduced (gains electrons). Eventually, the positively charged hydrogen ions (H+) that were released from the glucose molecule combine with the negatively charged oxygen ion (O=) to form water (H2O).
As all the hydrogens are stripped off the glucose molecule, the remaining carbon and oxygen atoms are rearranged to form individual molecules of CO2. All the hydrogen originally a part of the glucose has been moved to the oxygen to form water. All the remaining carbon and oxygen atoms of the original glucose are now in the form of CO2. The energy released from this process is used to generate ATP (figure 6.2).
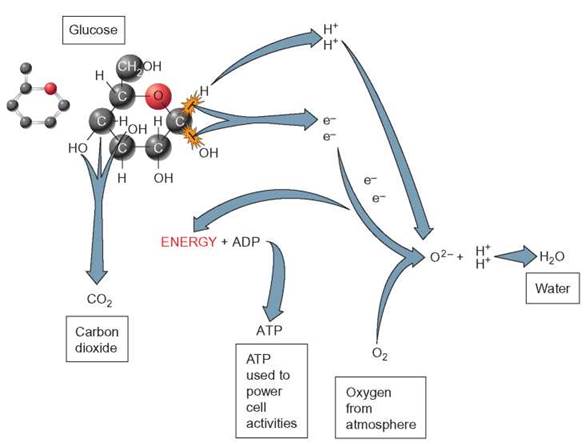
FIGURE 6.2. Aerobic Cellular Respiration and Oxidation-Reduction Reaction
During aerobic cellular respiration, a series of oxidation-reduction reactions takes place. When the electrons are removed (oxidation) from sugar, it is unable to stay together and breaks into smaller units. The reduction part of the reaction occurs when these electrons are attached to another molecule. In aerobic cellular respiration, the electrons are eventually picked up by oxygen and the negatively charged oxygen attracts two positively charged hydrogen ions (H+) to form water.
In cells, these reactions take place in a particular order and in particular places within the cell. In eukaryotic cells, the process of releasing energy from food molecules begins in the cytoplasm and is completed in the mitochondria. There are three distinct enzymatic pathways involved (figure 6.3): glycolysis, the Krebs cycle, and the electron-transport system.
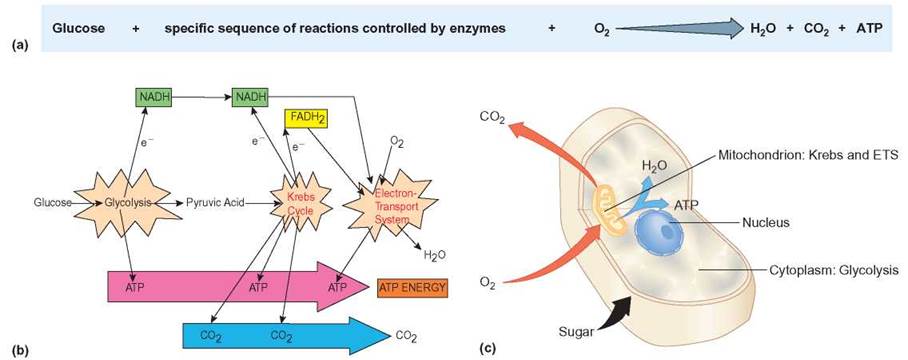
FIGURE 6.3. Aerobic Cellular Respiration: Overview
(a) This sequence of reactions in the aerobic oxidation of glucose is an overview of the energy-yielding reactions of a cell. (b) Glycolysis, the Krebs cycle, and the electron-transport system (ETS) are each a series of enzyme-controlled reactions that extract energy from the chemical bonds in a glucose molecule. During glycolysis, glucose is split into pyruvic acid and ATP and electrons are released. During the Krebs cycle, pyruvic acid is further broken down to carbon dioxide with the release of ATP and the release of electrons. During the electron-transport system, oxygen is used to accept electrons, and water and ATP are produced. (c) Glycolysis takes place in the cytoplasm of the cell. Pyruvic acid enters mitochondria, where the Krebs cycle and electron-transport system (ETS) take place.
Glycolysis
Glycolysis (glyco = sugar; lysis = to split) is a series of enzyme-controlled, anaerobic reactions that takes place in the cytoplasm of cells, which results in the breakdown of glucose with the release of electrons and the formation of ATP. During glycolysis, the 6-carbon sugar glucose is split into two smaller, 3-carbon molecules, which undergo further modification to form pyruvic acid or pyruvate.1 Enough energy is released to produce two ATP molecules. Some of the bonds holding hydrogen atoms to the glucose molecule are broken, and the electrons are picked up by electron carrier molecules (NAD+) and transferred to a series of electron-transfer reactions known as the electron-transport system (ETS).
The Krebs Cycle
The Krebs cycle is a series of enzyme-controlled reactions that takes place inside the mitochondrion, which completes the breakdown of pyruvic acid with the release of carbon dioxide, electrons, and ATP. During the Krebs cycle, the pyruvic acid molecules produced from glycolysis are further broken down. During these reactions, the remaining hydrogens are removed from the pyruvic acid, and their electrons are picked up by the electron carriers NAD+ and FAD. These electrons are sent to the electron-transport system. A small amount of ATP is also formed during the Krebs cycle. The carbon and oxygen atoms that are the remains of the pyruvic acid molecules are released as carbon dioxide (CO2).
The Electron-Transport System (ETS)
The electron-transport system (ETS) is a series of enzyme-controlled reactions that converts the kinetic energy of hydrogen electrons to ATP. The electrons are carried to the electron-transport system from glycolysis and the Krebs cycle as NADH and FADH2. The electrons are transferred through a series of oxidation-reduction reactions involving enzymes until eventually the electrons are accepted by oxygen atoms to form oxygen ions (O=). During this process, a great deal of ATP is produced. The ATP is formed as a result of a proton gradient established when the energy of electrons is used to pump protons across a membrane (refer to chapter 5). The subsequent movement of protons back across the membrane results in ATP formation. The negatively charged oxygen atoms attract two positively charged hydrogen ions to form water (H2O).
Aerobic respiration can be summarized as follows. Glucose enters glycolysis and is broken down to pyruvic acid, which enters the Krebs cycle, where the pyruvic acid molecules are further dismantled. The remains of the pyruvic acid molecules are released as carbon dioxide. The electrons and hydrogen ions released from glycolysis and the Krebs cycle are transferred as NADH and FADH2to the electron-t ransport system, where the electrons are transferred to oxygen available from the atmosphere. When hydrogen ions attach to oxygen ions, water is formed. ATP is formed during all three stages of aerobic cellular respiration, but most comes from the electron- transfer system.
6.2. CONCEPT REVIEW
4. Aerobic cellular respiration occurs in three stages. Name these and briefly describe what happens in each stage.
5. Which cellular organelle is involved in the process of aerobic cellular respiration?
_______________________________________________________
1Several different ways of naming organic compounds have been used over the years. For our purposes, pyruvic acid and pyruvate are really the same basic molecule although technically, pyruvate is what is left when pyruvic acid has lost its hydrogen ion: pyruvic acid → H+ + pyruvate. You also will see terms such as lactic acid and lactate and citric acid and citrate and many others used in a similar way.