CONCEPTS IN BIOLOGY
PART II. CORNERSTONES: CHEMISTRY, CELLS, AND METABOLISM
2. The Basics of Life
2.2. The Nature of Matter
The idea that substances are composed of very small particles goes back to early Greek philosophers. During the fifth century B.c., Democritus wrote that matter was empty space filled with tremendous numbers of tiny, indivisible particles called atoms. (The word atom is from the Greek word meaning uncuttable.)
Structure of the Atom
Recall from chapter 1 that atoms are the smallest units of matter that can exist alone. Elements are fundamental chemical substances made up of collections of only one kind of atom. For example, hydrogen (the most basic element), helium, lead, gold, potassium, and iron are all elements. There are over 100 elements. To understand how the atoms of various elements differ from each other, we need to look at the structure of atoms (How Science Works 2.1).
Atoms are constructed of three major subatomic particles: neutrons, protons, and electrons. A neutron is a heavy subatomic (units smaller than an atom) particle that does not have a charge; it is located in the central core of each atom. The central core is called the atomic nucleus. The mass of the atom is concentrated in the atomic nucleus. A proton is a heavy subatomic particle that has a positive charge; it is also located in the atomic nucleus. An electron is a light subatomic particle with a negative electrical charge that moves about outside the atomic nucleus in regions known as energy levels (figure 2.3). An energy level is a region of space surrounding the atomic nucleus that contains electrons with certain amounts of energy. The number of electrons an atom has determines the space, or volume, an atom takes up.
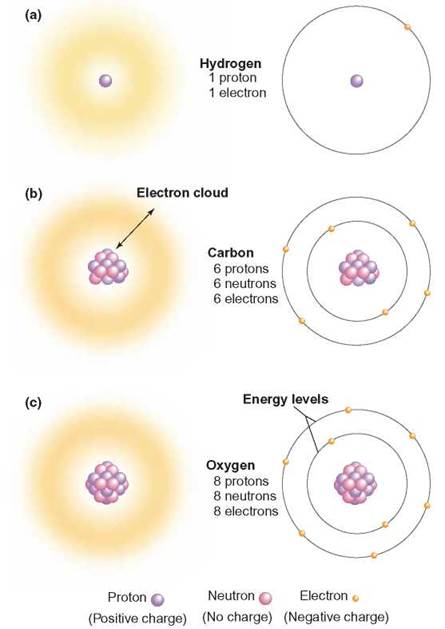
FIGURE 2.3. Atomic Structure
All the atoms of an element have the same number of protons and determine the element’s uniqueness. The fuzzy areas in the left column show how the electrons create the volume of the atoms. Those in the right column show individual electrons where they might be in their energy levels.
All the atoms of an element have the same number of protons. The number of protons determines the element’s identity. For example, carbon always has 6 protons; no other element has that number. Oxygen always has 8 protons. The atomic number of an element is the number of protons in an atom of that element; therefore, each element has a unique atomic number. Because oxygen has 8 protons, its atomic number is 8. The mass of a proton is 1.67 x 10-24 grams. Because this is an extremely small mass and is awkward to express, 1 proton is said to have a mass of 1 atomic mass unit (abbreviated as AMU) (table 2.1).
TABLE 2.1. Comparison of Atomic Particles
|
Protons |
Neutrons |
Electrons |
|
|
Location |
Nucleus |
Nucleus |
Outside nucleus |
|
Charge |
Positive (+) |
None (neutral) |
Negative (—) |
|
Number present |
Identical to atomic number |
Atomic weight minus atomic number |
Equal to number of protons |
|
Mass |
1 AMU |
1 AMU |
1/1,836 AMU |
Elements May Vary in Neutrons but Not Protons
Although all atoms of the same element have the same number of protons and electrons, they do not always have the same number of neutrons. In the case of oxygen, over 99% of the atoms have 8 neutrons, but others have more or fewer neutrons. Each atom of the same element with a different number of neutrons is called an isotope of that element. Since neutrons have a mass very similar to that of protons, isotopes that have more neutrons have a greater mass than those that have fewer neutrons.
Elements occur in nature as a mixture of isotopes. The atomic weight of an element is an average of all the isotopes present in a mixture in their normal proportions. For example, of all the hydrogen isotopes on Earth, 99.985% occur as an isotope without a neutron and 0.015% as an isotope with 1 neutron. There is a third isotope with 2 neutrons, and is even more rare. When the math is done to account for the relative amounts of these three isotopes of hydrogen, the atomic weight turns out to be 1.0079 AMU.
The sum of the number of protons and neutrons in the nucleus of an atom is called the mass number. Mass numbers are used to identify isotopes. The most common isotope of hydrogen has 1 proton and no neutrons. Thus, its mass number is 1 (1 proton + 0 neutrons = 1) also called protium. A hydrogen atom with 1 proton and 1 neutron has a mass number of 1 + 1, or 2, and is referred to as hydrogen-2, also called deuterium. A hydrogen atom with 1 proton and 2 neutrons has a mass number of 1 + 2, or 3, and is referred to as hydrogen-3, also called tritium (figure 2.4). All three isotopes of hydrogen are found on Earth, but the most frequently occurring has 1 AMU and is commonly called hydrogen. Most scientists use the term hydrogen in a generic sense (i.e., the term is not specific but might refer to any or all of these isotopes).
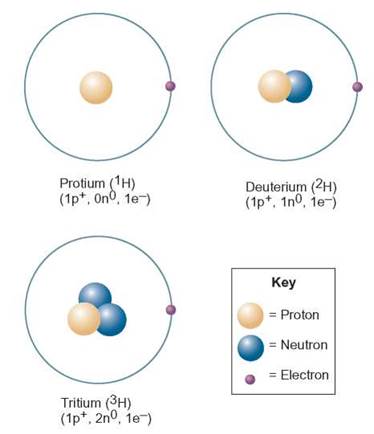
FIGURE 2.4. Isotopes of Hydrogen
Isotopes vary in the number of neutrons they contain in the atomic nucleus. Take a look at the three isotopes of hydrogen shown here and compare the nuclei. Since the mass of an atom is located in the nucleus, these three isotopes will differ in their weights.
Subatomic Particles and Electrical Charge
Subatomic particles were named to reflect their electrical charge. Protons have a positive (+) electrical charge. Neutrons are neutral because they lack an electrical charge (0). Electrons have a negative (—) electrical charge. Because positive and negative particles are attracted to one another, electrons are held near the nucleus. However, their kinetic energy (motion) keeps them from combining with the nucleus. The overall electrical charge of an atom is neutral (0) because the number of protons (positively charged) equals the number of electrons (negatively charged). For instance, hydrogen, with 1 proton, has 1 electron; carbon, with 6 protons, has 6 electrons. You can determine the number of either of these two particles in an atom if you know the number of the other particle.
Scientists’ understanding of the structure of an atom has changed since the concept was first introduced. At one time, people thought of atoms as miniature solar systems, with the nucleus in the center and electrons in orbits, like satellites, around the nucleus. However, as more experimental data were gathered and interpreted, a new model was formulated.
The Position of Electrons
In contrast to the “solar system” model, electrons are now believed to occupy certain areas around the nucleus—the energy levels. Each energy level contains electrons moving at approximately the same speed; therefore, electrons of a given level have about the same amount of kinetic energy. Each energy level is numbered in increasing order, with energy level 1 containing electrons closest to the nucleus, with the lowest amount of energy. The electrons in energy level 2 have more energy and are farther from the nucleus than those found in energy level 1. Electrons in energy level 3 having electrons with even more energy are still farther from the nucleus than those in level 2 and so forth.
Electrons do not encircle the atomic nucleus in twodimensional paths. Some move around the atomic nucleus in a three-dimensional region that is spherical, forming cloudlike or fuzzy layers about the nucleus. Others move in a manner that resembles the figure 8, forming fuzzy regions that look like dumbbells or hourglasses (figure 2.5).
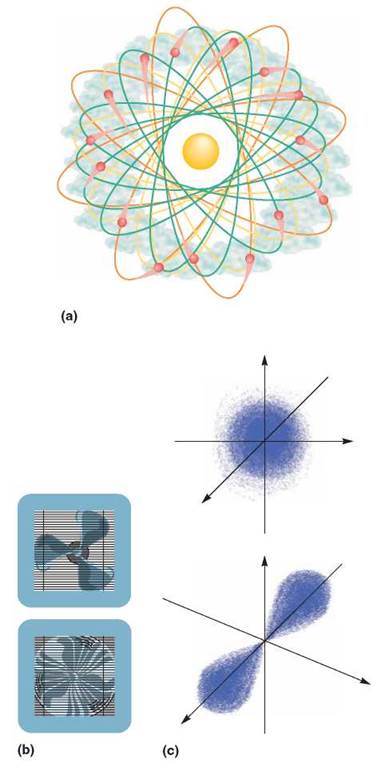
FIGURE 2.5. The Electron Cloud
Electrons are moving around the nucleus so fast that they can be thought of as forming a cloud around it, rather than an orbit or a single track. (a) You might think of the electron cloud as hundreds of photographs of an atom. Each photograph shows where an electron was at the time the picture was taken. However, when the next picture is taken, the electron has moved to a different place. In effect, an electron appears to be everyplace in its energy level at the same time, just as the fan blade of a window fan is everywhere at once when it is running. (b) No matter where you stick your finger in the fan, you will be touched by the moving blade. Although we are able to determine where an electron is at a given time, we do not know the exact path it uses to go from one place to another. (c) This is a better way to represent the positions of electrons in spherical and hourglass configurations.
The first energy level is full when it has 2 electrons. The second energy level is full when it has 8 electrons; the third energy level, 8; and so forth (table 2.2). Also note in table 2.2 that, for some of the atoms (He, Ne, Ar), the outermost energy level contains the maximum number of electrons it can hold. Elements such as He and Ne, with filled outer energy levels, are particularly stable.
TABLE 2.2. Number of Electrons in Energy Level
|
Element |
Symbol |
Atomic Number |
Energy Level 1 |
Energy Level 2 |
Energy Level 3 |
Energy Level 4 |
|
Hydrogen |
H |
1 |
1 |
|||
|
Helium |
He |
2 |
2 |
|||
|
Carbon |
C |
6 |
2 |
4 |
||
|
Nitrogen |
N |
7 |
2 |
5 |
||
|
Oxygen |
O |
8 |
2 |
6 |
||
|
Neon |
Ne |
10 |
2 |
8 |
||
|
Sodium |
Na |
11 |
2 |
8 |
1 |
|
|
Magnesium |
Mg |
12 |
2 |
8 |
2 |
|
|
Phosphorus |
P |
15 |
2 |
8 |
5 |
|
|
Sulfur |
S |
16 |
2 |
8 |
6 |
|
|
Chlorine |
Cl |
17 |
2 |
8 |
7 |
|
|
Argon |
Ar |
18 |
2 |
8 |
8 |
|
|
Potassium |
K |
19 |
2 |
8 |
8 |
1 |
|
Calcium |
Ca |
20 |
2 |
8 |
8 |
2 |
All atoms have a tendency to seek such a stable, filled outer energy level arrangement, a tendency referred to as the octet (8) rule. (Hydrogen and helium are exceptions to this rule and have a filled outer energy level when they have 2 electrons.) The rule states that atoms attempt to acquire an outermost energy level with 8 electrons through processes called chemical reactions. Because elements such as He and Ne have full outermost energy levels under ordinary circumstances, they do not normally undergo chemical reactions. These elements are referred to as inert or noble (implying that they are too special to interact with other elements). Atoms of other elements have outer energy levels that are not full. For example, H, C, Mg, and Ca will undergo reactions to fill their outermost energy level in order to become stable. it is important for chemists and biologists to focus on electrons in the outermost energy level, because it is these electrons that are involved in the chemical activities of all life.
2.2. CONCEPT REVIEW
3. What is meant by an “energy level”?
4. Define subatomic particle.
5. Why do chemicals undergo reactions?
HOW SCIENCE WORKS 2.1
The Periodic Table of the Elements
Traditionally, the elements have been represented in a shorthand form by letters called chemical symbols. The table that displays these symbols is called periodic because the properties of the elements recur periodically (at regular intervals) when the elements are listed in order of their size. The table has horizontal rows of elements called periods. The vertical columns are called families. The periods and families consist of squares, with each element having its own square in a specific location. This arrangement has a meaning, both about atomic structure and about chemical functions. The periods are numbered from 1 to 7 on the left side. Period 1, for example, has only two elements: H (hydrogen) and He (helium). Period 2 starts with Li (lithium) and ends with Ne (neon). The two rows at the bottom of the table are actually part of periods 6 and 7 (between atomic numbers 57 and 72, 89 and 104). They are moved so that the table is not so wide.
Families are identified with Roman numerals and letters at the top of each column. Family IIA, for example, begins with Be (beryllium) at the top and has Ra (radium) at the bottom. The A families are in sequence from left to right. The B families are not in sequence, and one group contains more elements than the others. The elements in vertical columns have similar arrangements of electrons, and that structure is responsible for the chemical properties of an element. Don't worry—you will not have to memorize the entire table. The 11 main elements comprising living things have the chemical symbols C, H, O, P, K, I, N, S, Ca, Fe, and Mg. (A mnemonic trick to help you remember them is CHOPKINS CaFe, Mighty good!).
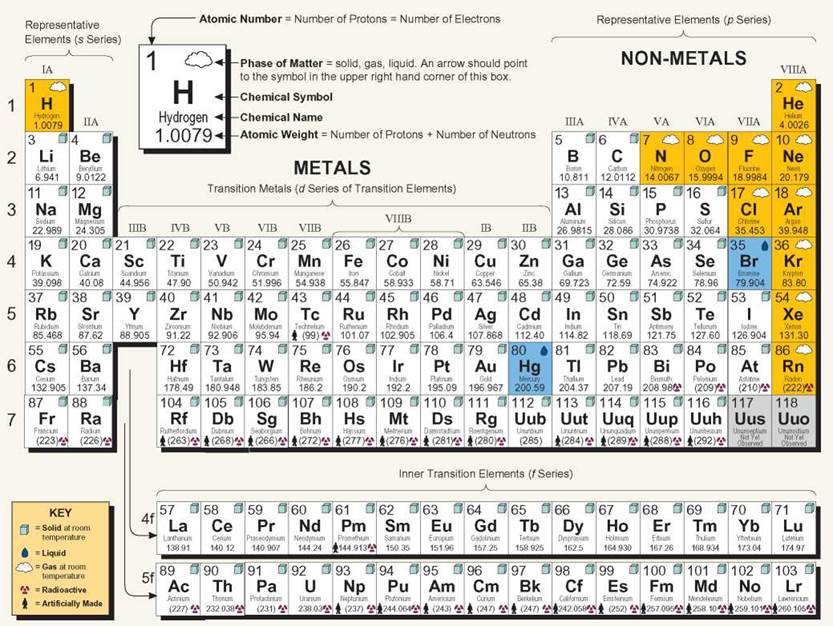
Periodic Table of the Elements
The table provides information about all the known elements. Notice that the atomic weights of the elements increase as you read left to right along the periods. Reading top to bottom in a family gives you a glimpse of a group of elements that have similar chemical properties.