CONCEPTS IN BIOLOGY
PART II. CORNERSTONES: CHEMISTRY, CELLS, AND METABOLISM
2. The Basics of Life
2.3. The Kinetic Molecular Theory and Molecules
Greek philosopher Aristotle (384-322 B.c.) rejected the idea of atoms. He believed that matter was continuous and made up of only four parts: earth, air, fire, and water. Aristotle’s belief about matter predominated through the 1600s. Galileo and Newton, however, believed the ideas about matter being composed of tiny particles, or atoms, because this theory seemed to explain matter’s behavior. Widespread acceptance of the atomic model did not occur, however, until strong evidence was developed through the science of chemistry in the late 1700s and early 1800s. The experiments finally led to a collection of assumptions about the small particles of matter and the space around them; these assumptions came to be known as the kinetic molecular theory. The kinetic molecular theory states that all matter is made up of tiny particles, which are in constant motion.
The Formation of Molecules
Because atoms tend to fill their outer energy levels, they often interact with other atoms. Recall from chapter 1 that a molecule is the smallest particle of a chemical compound and is a definite and distinct, electrically neutral group of bonded atoms. Some atoms, such as oxygen, hydrogen, and nitrogen, bond to form diatomic (di = two) molecules. In our atmosphere, these elements are found as the gases H2, O2, and N2. The subscript indicates the number of atoms of an element in a single molecule of a substance. Other elements are not normally diatomic but exist as single, or monatomic (mon = one), units—for example, the gases helium (He) and neon (Ne). These chemical symbols, or initials, indicate a single atom of that element.
Two or more different kinds of atoms can combine, forming a compound. A compound is a chemical substance made up of atoms of two or more elements combined in a specific ratio and arrangement. The attractive forces that hold the atoms of a molecule together are called chemical bonds. Molecules can consist of two or more atoms of the same element (such as O2 or N2) or of specific numbers of atoms of different elements (figure 2.6).
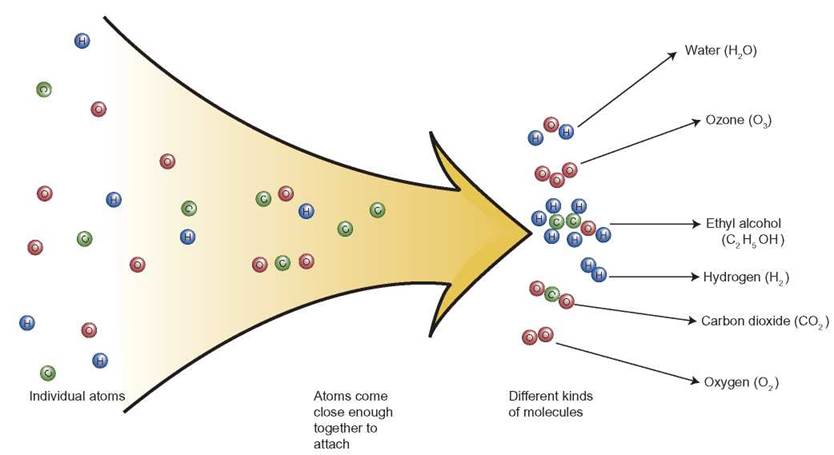
FIGURE 2.6 The Formation of Molecules
This figure shows how atoms of carbon, hydrogen and oxygen come together to form different kinds of molecules. If two atoms of hydrogen attach to one of oxygen, the result is a molecule of water (H2O). Depending on the kinds of atoms involved and their numbers, other kinds of molecules, compounds, can be formed.
The formula of a compound describes what elements it contains (as indicated by a chemical symbol) and in what proportions they occur (as indicated by the subscript number). For example, pure water is composed of two atoms of hydrogen and one atom of oxygen. It is represented by the chemical formula H2O. The subscript “2” indicates two atoms of the element hydrogen, and the symbol for oxygen without a subscript indicates that there is only 1 atom of oxygen present in this molecule.
2.3. CONCEPT REVIEW
6. What is the difference between an atom and an element?
7. What is the difference between a molecule and a compound?