Biology For Dummies
Part I Biology Basics
Chapter 5
Acquiring Energy to Run the Motor
In This Chapter
Recognizing the important role energy plays in organisms
Making food with photosynthesis
Metabolizing food for energy through cellular respiration
Counting calories
Just like you need to put gas in your car’s engine so your car can move, you need to put food in your body so it can function. And you’re not alone. Every person, as well as every other living thing, needs to “fill its tank” with matter and energy in the form of food. Food molecules are used to build the molecules that make up cells and are broken down to release energy to cells so they can grow and maintain themselves. Animals obtain their food by eating plants and other animals, whereas plants make their own food. In this chapter, we present some facts about the various types of energy and how they’re transferred. We also demonstrate why cells need energy and take a look at how cells obtain and then store energy and matter.
What’s Energy Got to Do with It?
Whether you realize it or not, you use energy every day to cook your food, brighten your home, and run your appliances. Chances are, however, if you were asked to state what energy is, you might have some trouble. Most people have an idea of energy as something you need to do things, but they don’t really know what energy is. The funny thing is that physicists, who spend a lot of time studying energy, define energy in exactly the way most people think about it: Energy is something that allows work to be done.
You can probably think of many kinds of energy in your life — electricity, heat, light, chemical (like gasoline). Although they may seem very different, the kinds of energy you can think of represent the two main types of energy:
Potential energy: This is the energy that’s stored in something because of the way it’s arranged or structured. Energy in a battery, water behind a dam, and a stretched rubber band that’s about to be released are all examples of potential energy. Food and gasoline also contain potential energy called chemical potential energy (energy that’s stored in the bonds of molecules).
Kinetic energy: This is the energy of motion. Light, heat, and moving objects all contain kinetic energy.
The following sections get you acquainted with the rules surrounding energy. They also explain how the cells of living things use and transfer energy, as well as how they obtain it (here’s a hint: it’s all about food).
Looking at the rules regarding energy
Energy has three specific rules that are helpful to know so you can better understand how organisms use it:
Energy can’t be created or destroyed. The electricity that people get from hydroelectric power (or coal-burning power plants, wind turbines, or solar panels) isn’t created from nothing. It’s actually transferred from some other kind of energy. And when people use, say, electricity, that energy doesn’t disappear. Instead, it becomes other kinds of energy, such as light or heat.
 The idea that energy can’t be created or destroyed is known as the First Law of Thermodynamics.
The idea that energy can’t be created or destroyed is known as the First Law of Thermodynamics.
Energy is transferred when it moves from one place to another. To understand this rule, picture a flowing river that’s used as a source of hydroelectric power. Energy from the moving river is transferred first to a spinning turbine, then to flowing electrons in power lines, and finally to the lights shining in customers’ homes.
Energy is transformed when it changes from one form to another. Again, think about a hydroelectric power plant. The potential energy of the water behind the plant’s dam is transformed first into the kinetic energy of moving water, then the kinetic energy of a spinning turbine, and finally the kinetic energy of moving electrons.
Metabolizing molecules
Organisms follow the rules of physics and chemistry, and the human body is no exception. The First Law of Thermodynamics (explained in the preceding section) applies to your metabolism, which is all the chemical reactions occurring in your cells at one time.
Two types of chemical reactions can occur as an organism metabolizes molecules:
Anabolic reactions: This type of reaction builds molecules. Specifically, small molecules are combined into large molecules for repair, growth, or storage.
Catabolic reactions: This type of reaction breaks down molecules to release their stored energy.
 During chemical reactions, atoms receive new bonding partners, and energy may be transferred. (For more on molecules, atoms, and chemical bonds, flip to Chapter 3.)
During chemical reactions, atoms receive new bonding partners, and energy may be transferred. (For more on molecules, atoms, and chemical bonds, flip to Chapter 3.)
 Each type of food molecule you’re familiar with — carbohydrates, proteins, and fats — are large molecules that can be broken down into smaller subunits. Complex carbohydrates, also called polysaccharides, break down into simple sugars called monosaccharides; proteins break down into amino acids; and fats and oils break down into glycerol and fatty acids. After cells break large food molecules down into their subunits, they can more easily reconnect the subunits and reform them into the specific molecules that they need.
Each type of food molecule you’re familiar with — carbohydrates, proteins, and fats — are large molecules that can be broken down into smaller subunits. Complex carbohydrates, also called polysaccharides, break down into simple sugars called monosaccharides; proteins break down into amino acids; and fats and oils break down into glycerol and fatty acids. After cells break large food molecules down into their subunits, they can more easily reconnect the subunits and reform them into the specific molecules that they need.
Transferring energy with ATP
Cells transfer energy between anabolic and catabolic reactions by using an energy middleman — adenosine triphosphate (or ATP for short). Energy from catabolic reactions is transferred to ATP, which then provides energy for anabolic reactions.
ATP has three phosphates attached to it (tri- means “three,” so triphosphate means “three phosphates”). When ATP supplies energy to a process, one of its phosphates gets transferred to another molecule, turning ATP into adenosine diphosphate (ADP). Cells re-create ATP by using energy from catabolic reactions to reattach a phosphate group to ADP. Cells constantly build and break down ATP, creating the ATP/ADP cycle shown in Figure 5-1.
Figure 5-1: The ATP/ADP cycle.
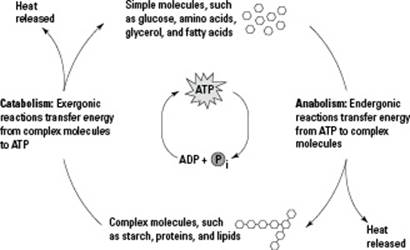
Cells have large molecules that contain stored energy, but when they’re busy doing work, they need a handy source of energy. That’s where ATP comes in. Cells keep ATP on hand to supply energy for all the work that they do.
 Think of ATP like cash in your pocket. You may have money deposited in the bank, but that money isn’t always easy to get, which is why you keep some cash in your pocket to quickly buy what you need. After you spend all of your cash, you have to go back to the bank or an ATM to get more. For living things, the energy stored in large molecules is like money in the bank. Cells break down ATP just like you spend your cash. Then, when cells need more ATP, they have to go back to the bank of large molecules and break some more down.
Think of ATP like cash in your pocket. You may have money deposited in the bank, but that money isn’t always easy to get, which is why you keep some cash in your pocket to quickly buy what you need. After you spend all of your cash, you have to go back to the bank or an ATM to get more. For living things, the energy stored in large molecules is like money in the bank. Cells break down ATP just like you spend your cash. Then, when cells need more ATP, they have to go back to the bank of large molecules and break some more down.
Consuming food for matter and energy
Food molecules — in the form of proteins, carbohydrates, and fats — provide the matter and energy that every living thing needs to fuel anabolic and catabolic reactions and create ATP (for more on matter and molecules, see Chapter 3).
Organisms need matter to build their cells so they can grow, repair themselves, and reproduce. Imagine that you scrape your knee and actually remove a fair amount of skin. Your body repairs the damage by building new skin cells to cover the scraped area. Just like a person who builds a house needs wood or bricks, your body needs molecules to build new cells (head to Chapter 4 for the full scoop on cells).
Organisms need energy so they can move, build new materials, and transport materials around their cells. These activities are all examples of cellular work, the energy-requiring processes that occur in cells. When you walk up stairs, the muscle cells in your legs contract, and each contraction uses some energy. But the activities you decide to engage in aren’t the only things that require energy. Your individual cells also need energy to do their work.
 Food is a handy package that contains two things every organism needs: matter and energy.
Food is a handy package that contains two things every organism needs: matter and energy.
Finding food versus producing your own
All organisms need food, but there’s one major difference in how they approach this problem: Some organisms, such as plants, can make their own food; other organisms, like you, have to eat other organisms to obtain their food. Biologists have come up with two separate categories to highlight this difference in how living things obtain their food:
Autotrophs can make their own food. Auto means “self,” and troph means “feed,” so autotrophs are self-feeders. Plants, algae, and green bacteria are all examples of autotrophs.
Heterotrophs have to eat other organisms to get their food. Hetero means “other,” so heterotrophs are quite literally other-feeders. Animals, fungi, and most bacteria are examples of heterotrophs.
Although you may think that obtaining food is as easy as heading to the supermarket, pulling up to a drive-through window, or meeting the delivery guy at the front door, acquiring nutrients is actually a metabolic process. More specifically, food is made through one process and broken down through another. These processes are as follows:
Photosynthesis: Only autotrophs such as plants, algae, and green bacteria engage in photosynthesis, a process that consists of using energy from the Sun, carbon dioxide from the air, and water from the soil to make sugars. (The carbon dioxide provides the matter plants need for food building.) When plants remove hydrogen atoms from water to use in the sugars, they release oxygen as waste.
Cellular respiration: Both autotrophs and heterotrophs do cellular respiration, a process that uses oxygen to help break down food molecules such as sugars. The energy stored in the bonds of the food molecules is transferred to ATP. As the energy is transferred to the cells, the matter from the food molecules is released as carbon dioxide and water.
 If you think about it, photosynthesis and cellular respiration are really the opposites of each other. Photosynthesis consumes carbon dioxide and water, producing food and oxygen. Cellular respiration consumes food and oxygen, producing carbon dioxide and water. Scientists write the big picture view of both processes as the following equations:
If you think about it, photosynthesis and cellular respiration are really the opposites of each other. Photosynthesis consumes carbon dioxide and water, producing food and oxygen. Cellular respiration consumes food and oxygen, producing carbon dioxide and water. Scientists write the big picture view of both processes as the following equations:
Photosynthesis:
6 CO2 + 6 H2O + Light Energy → C6H12O6 + 6 O2
Cellular respiration:
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + Usable Energy
 Don’t fall for the idea that only heterotrophs such as animals engage in cellular respiration. Autotrophs such as plants do it too. Think of it like this: Photosynthesis is a food-making pathway that autotrophs use to store matter and energy for later. So, a plant doing photosynthesis is like you packing a lunch for yourself. There wouldn’t be much point in packing the lunch if you weren’t going to eat it later, right? The same is true for a plant. It does photosynthesis to store matter and energy. When it needs that matter and energy, it uses cellular respiration to “unpack” its food.
Don’t fall for the idea that only heterotrophs such as animals engage in cellular respiration. Autotrophs such as plants do it too. Think of it like this: Photosynthesis is a food-making pathway that autotrophs use to store matter and energy for later. So, a plant doing photosynthesis is like you packing a lunch for yourself. There wouldn’t be much point in packing the lunch if you weren’t going to eat it later, right? The same is true for a plant. It does photosynthesis to store matter and energy. When it needs that matter and energy, it uses cellular respiration to “unpack” its food.
Photosynthesis: Using Sunlight, Carbon Dioxide, and Water to Make Food
Autotrophs such as plants combine matter and energy to make food in the form of sugars. With those sugars, plus some nitrogen and minerals from the soil, autotrophs can make all the types of molecules they need to build their cells. The chemical formula for glucose, the most common type of sugar found in cells, is C6H12O6. To build glucose, autotrophs need carbon, hydrogen, and oxygen atoms, plus energy to combine them into sugar.
The carbon and oxygen for the sugars come from carbon dioxide in the Earth’s atmosphere.
The hydrogen for the sugars comes from water found in the soil.
The energy to build the sugars comes from the Sun (but only in autotrophs that use photosynthesis).
 A common misconception is that plants get the matter they need to grow from the soil. This seems like a perfectly logical idea given that plants grow with their roots stuck in the ground. However, some very careful scientific observations by a Belgian scientist named Jean Baptiste van Helmont showed that a tree that gained 169 pounds in mass as it grew took only 2 ounces of dry material from the soil (not counting water). This experiment proved that plants don’t take lots of material from the soil. Instead, they get most of the matter they need to grow from the carbon dioxide in the air. This idea may be more difficult to believe because air, including carbon dioxide, doesn’t seem like much of anything, but scientists have proven that it’s correct. Plants collect a lot of carbon dioxide molecules (CO2) and combine them with water molecules (H2O) to build sugars such as glucose (C6H12O6). Plants get the water they need, plus some small amounts of minerals such as nitrogen, from the soil.
A common misconception is that plants get the matter they need to grow from the soil. This seems like a perfectly logical idea given that plants grow with their roots stuck in the ground. However, some very careful scientific observations by a Belgian scientist named Jean Baptiste van Helmont showed that a tree that gained 169 pounds in mass as it grew took only 2 ounces of dry material from the soil (not counting water). This experiment proved that plants don’t take lots of material from the soil. Instead, they get most of the matter they need to grow from the carbon dioxide in the air. This idea may be more difficult to believe because air, including carbon dioxide, doesn’t seem like much of anything, but scientists have proven that it’s correct. Plants collect a lot of carbon dioxide molecules (CO2) and combine them with water molecules (H2O) to build sugars such as glucose (C6H12O6). Plants get the water they need, plus some small amounts of minerals such as nitrogen, from the soil.
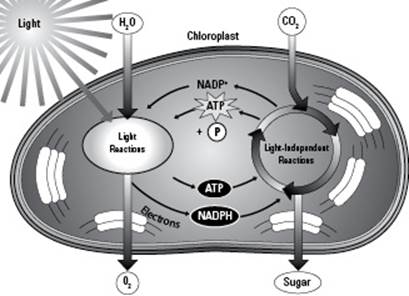
 Photosynthesis occurs in two main steps (Figure 5-2 depicts both in action):
Photosynthesis occurs in two main steps (Figure 5-2 depicts both in action):
The light reactions of photosynthesis transform light energy into chemical energy. The chemical energy is stored in the energy carrier ATP.
The light-independent reactions of photosynthesis produce food. ATP from the light reactions supplies the energy needed to combine carbon dioxide (CO2) and water (H2O) to make glucose (C6H12O6).
The next sections delve deeper into the process of photosynthesis.
Figure 5-2: The two halves of photosynthesis, the light reactions and the light-independent reactions, are separate but linked.
Transforming energy from the ultimate energy source
The Sun is a perfect energy source — a nuclear reactor positioned at a safe distance from planet Earth. It contains all the energy you could ever need . . . if only you could capture it. Well, green bacteria figured out how to do just that more than 2.5 billion years ago, showing that photosynthetic autotrophs were way ahead of humans on this one.
Plants, algae, and green bacteria use pigments to absorb light energy from the Sun. You’re probably most familiar with the pigment chlorophyll, which colors the leaves of plants green. The chloroplasts in plant cells contain lots of chlorophyll in their membranes so they can absorb light energy (see Chapter 4 for more on chloroplasts).
During the light reactions of photosynthesis, chloroplasts absorb light energy from the Sun and then transform it into the chemical energy stored in ATP. When the light energy is absorbed, it splits water molecules. The electrons from the water molecules help with the energy transformation from light energy to chemical energy in ATP. Plants release the oxygen from the water molecules as waste, producing the oxygen (O2) that you breathe.
Putting matter and energy together
Plants use the energy in ATP (which is a product of the light reactions) to combine carbon dioxide molecules and water molecules to create glucose during the light-independent reactions. To make glucose, plants first take carbon dioxide out of the air through a process called carbon fixation (taking carbon dioxide and attaching it to a molecule inside the cell). They then use the energy from the ATP and the electrons that came from water to convert the carbon dioxide to sugar.
 The light-independent reactions form a metabolic cycle that’s known as the Calvin-Benson cycle (named after the scientists who discovered it).
The light-independent reactions form a metabolic cycle that’s known as the Calvin-Benson cycle (named after the scientists who discovered it).
 As their name indicates, the light-independent reactions of photosynthesis don’t need direct sunlight to occur. However, plants need the products of the light reactions to run the light-independent reactions, so really, the light-independent reactions can’t happen if the light reactions can’t happen.
As their name indicates, the light-independent reactions of photosynthesis don’t need direct sunlight to occur. However, plants need the products of the light reactions to run the light-independent reactions, so really, the light-independent reactions can’t happen if the light reactions can’t happen.
When plants have made more glucose than they need, they store their excess matter and energy by combining glucose molecules into larger carbohydrate molecules, such as starch. When necessary, plants can break down the starch molecules to retrieve glucose for energy or to create other compounds, such as proteins and nucleic acids (with added nitrogen taken from the soil) or fats (many plants, such as olives, corn, peanuts, and avocados, store matter and energy in oils).
Cellular Respiration: Using Oxygen to Break Down Food for Energy
Autotrophs and heterotrophs do cellular respiration to break down food to transfer the energy from food to ATP. The cells of animals, plants, and many bacteria use oxygen to help with the energy transfer during cellular respiration; in these cells, the type of cellular respiration that occurs is aerobic respiration (aerobic means “with air”).
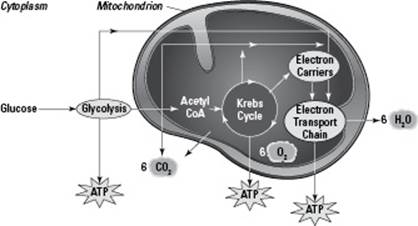
 Three separate pathways combine to form the process of cellular respiration (you can see them all in action in Figure 5-3). The first two, glycolysis and the Krebs cycle, break down food molecules. The third pathway, oxidative phosphorylation, transfers the energy from the food molecules to ATP. Here are the basics of how cellular respiration works:
Three separate pathways combine to form the process of cellular respiration (you can see them all in action in Figure 5-3). The first two, glycolysis and the Krebs cycle, break down food molecules. The third pathway, oxidative phosphorylation, transfers the energy from the food molecules to ATP. Here are the basics of how cellular respiration works:
During glycolysis, which occurs in the cytoplasm of the cell, cells break glucose down into pyruvate, a three-carbon compound. After glycolysis, pyruvate is broken down into a two-carbon molecule called acetyl-coA.
After pyruvate is converted to acetyl-coA, cells use the Krebs cycle (which occurs in the matrix of the mitochondrion) to break down acetyl-coA into carbon dioxide.
During oxidative phosphorylation, which occurs in the inner membrane or cristae of the mitochondrion), cells transfer energy from the breakdown of food to ATP.
Figure 5-3: An overview of cellular respiration.
For a more in-depth look at cellular respiration, check out the following sections.
 Cellular respiration is different from plain ol’ respiration. Respiration, which is more commonly referred to as breathing, is the physical act of inhaling and exhaling. Cellular respiration is what happens inside cells when they use oxygen to transfer energy from food to ATP.
Cellular respiration is different from plain ol’ respiration. Respiration, which is more commonly referred to as breathing, is the physical act of inhaling and exhaling. Cellular respiration is what happens inside cells when they use oxygen to transfer energy from food to ATP.
Breaking down food
After the large molecules in food are broken down into their smaller subunits, the small molecules can be further broken down to transfer their energy to ATP. During cellular respiration, enzymes slowly rearrange the atoms in food molecules. Each rearrangement produces a new molecule in the pathway and can also produce other useful molecules for the cell. Some reactions
Release energy that can be transferred to ATP: Cells quickly use this ATP for cellular work, such as building new molecules.
Oxidize food molecules and transfer electrons and energy to coenzymes: Oxidation is the process that removes electrons from molecules; reduction is the process that gives electrons to molecules. During cellular respiration, enzymes remove electrons from food molecules and then transfer the electrons to the coenzymes nicotinamide adenine dinucleotide (NAD+) and flavin adenine dinucleotide (FAD). NAD+ and FAD receive the electrons as part of hydrogen (H) atoms, which change them to their reduced forms, NADH and FADH2. Next, NADH and FADH2 donate the electrons to the process of oxidative phosphorylation, which transfers energy to ATP.
 NAD+ and FAD act like electron shuttle buses for the cell. The empty buses, NAD+ and FAD, drive up to oxidation reactions and collect electron passengers. When the electrons get on the bus, the driver puts up the H sign to show that the bus is full. Then the full buses, NADH and FADH2, drive over to reactions that need electrons and let the passengers off. The buses are now empty again, so they drive back to another oxidation reaction to collect new passengers. During cellular respiration, the electron shuttle buses drive a loop between the reactions of glycolysis and the Krebs cycle (where they pick up passengers) to the electron transport chain (where they drop off passengers).
NAD+ and FAD act like electron shuttle buses for the cell. The empty buses, NAD+ and FAD, drive up to oxidation reactions and collect electron passengers. When the electrons get on the bus, the driver puts up the H sign to show that the bus is full. Then the full buses, NADH and FADH2, drive over to reactions that need electrons and let the passengers off. The buses are now empty again, so they drive back to another oxidation reaction to collect new passengers. During cellular respiration, the electron shuttle buses drive a loop between the reactions of glycolysis and the Krebs cycle (where they pick up passengers) to the electron transport chain (where they drop off passengers).
Release carbon dioxide (CO2): Cells return CO2 to the environment as waste, which is great for the autotrophs that require CO2 to produce the food that heterotrophs eat. (See how it’s all connected?)
Different kinds of food molecules enter cellular respiration at different points in the pathway. Cells break down simple sugars, such as glucose, in the first pathway — glycolysis. Cells use the second pathway, the Krebs cycle, for breaking down fatty acids and amino acids.
Following is a summary of how different molecules break down in the first two pathways of cellular respiration:
During glycolysis, glucose breaks down into two molecules of pyruvate. The backbone of glucose has six carbon atoms, whereas the backbone of pyruvate has three carbon atoms. During glycolysis, energy transfers result in a net gain of two ATP and two molecules of the reduced form of the coenzyme NADH.
Pyruvate is converted to acetyl-coA, which has two carbon atoms in its backbone. One carbon atom from pyruvate is released from the cell as CO2. For every glucose molecule broken down by glycolysis and the Krebs cycle, six CO2 molecules leave the cell as waste. (The conversion of pyruvate to acetyl-coA produces two molecules of carbon dioxide, and the Krebs cycle produces four.)
During the Krebs cycle, acetyl-coA breaks down into carbon dioxide (CO2). The conversion of pyruvate to acetyl-coA produces two molecules of NADH. Energy transfers during the Krebs cycle produce an additional six molecules of NADH, two molecules of FADH2, and two molecules of ATP.
Transferring energy to ATP
In the inner membranes of the mitochondria in your cells, hundreds of little cellular machines are busily working to transfer energy from food molecules to ATP. The cellular machines are called electron transport chains, and they’re made of a team of proteins that sits in the membranes transferring energy and electrons throughout the machines.
 The coenzymes NADH and FADH2 carry energy and electrons from glycolysis and the Krebs cycle to the electron transport chain. The coenzymes transfer the electrons to the proteins of the electron transport chain, which pass the electrons down the chain. Oxygen collects the electrons at the end of the chain. (If you didn’t have oxygen around at the end of the chain to collect the electrons, no energy transfer could occur.) When oxygen accepts the electrons, it also picks up protons (H+) and becomes water (H2O).
The coenzymes NADH and FADH2 carry energy and electrons from glycolysis and the Krebs cycle to the electron transport chain. The coenzymes transfer the electrons to the proteins of the electron transport chain, which pass the electrons down the chain. Oxygen collects the electrons at the end of the chain. (If you didn’t have oxygen around at the end of the chain to collect the electrons, no energy transfer could occur.) When oxygen accepts the electrons, it also picks up protons (H+) and becomes water (H2O).
 The proteins of the electron transport chain are like a bucket brigade that works by one person dumping a bucket full of water into the next person’s bucket. The buckets are the proteins, or electron carriers, and the water inside the buckets represents the electrons. The electrons get passed from protein to protein until they reach the end of the chain.
The proteins of the electron transport chain are like a bucket brigade that works by one person dumping a bucket full of water into the next person’s bucket. The buckets are the proteins, or electron carriers, and the water inside the buckets represents the electrons. The electrons get passed from protein to protein until they reach the end of the chain.
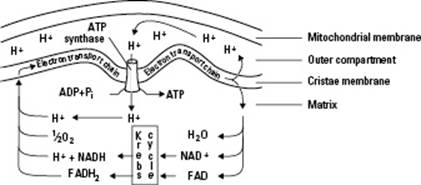
While electrons are transferred along the electron transport chain, the proteins use energy to move protons (H+) across the inner membranes of the mitochondria. They pile the protons up like water behind the “dam” of the inner membranes. These protons then flow back across the mitochondria’s membranes through a protein called ATP synthase that transforms the kinetic energy from the moving protons into chemical energy in ATP by capturing the energy in chemical bonds as it adds phosphate molecules to ADP.
The entire process of how ATP is made at the electron transport chain is called the chemiosmotic theory of oxidative phosphorylation and is illustrated in Figure 5-4.
 At the end of the entire process of cellular respiration, the energy transferred from glucose is stored in 36 to 38 molecules of ATP, which are available to be used for cellular work. (And boy do they get used quickly!)
At the end of the entire process of cellular respiration, the energy transferred from glucose is stored in 36 to 38 molecules of ATP, which are available to be used for cellular work. (And boy do they get used quickly!)
Figure 5-4: The events happening inside mitochondria, as described by the chemiosmotic theory.
Energy and Your Body
Your body takes in chemical potential energy when you eat food and then transfers the energy from that food to your cells. As you use the energy to do work, that energy is eventually transformed into heat energy that you transfer to your surroundings.
Energy can be measured in many different ways, but the energy in food is measured in calories. Basically, a calorie is a unit of measurement for heat energy. It takes 1 calorie to raise the temperature of 1 gram of water by 1 degree Celsius (not Fahrenheit). The calories that you count and see written on food packages are really kilocalories. (Kilo means “1,000,” so a kilocalorie is equal to 1,000 calories.) Kilocalories are represented by a capital C, whereas calories are represented by a lowercase c. From here on out, we use the term Calorie (with a capital C) to represent the kilocalories you’re familiar with from nutrition facts labels.
You can get an approximate measure of your basic energy needs by performing a simple calculation to determine your basal metabolic rate (BMR), the approximate number of Calories you need just to maintain your body’s minimum level of activity (breathing, blood pumping, digestion, and so on). Here’s how to calculate BMR:
1. Multiply your weight in pounds by 10.
2. Multiply your height in inches by 6.25.
3. Add these two numbers together.
4. Multiply your age by 5 and then subtract this number from the one you got in Step 3.
5. If you’re male, add 5 to the total you found in Step 4; if you’re female, subtract 161 from the total you found in Step 4.
If you exercise, you need to consume additional Calories to supply your body with the energy it needs for increased physical activity. Use the preceding calculation and Table 5-1 to figure out how many Calories you need to consume to maintain your lifestyle.
|
Table 5-1 Determining Caloric Need Based on Lifestyle |
|
|
If You’re . . . |
Multiply Your BMR by . . . |
|
Fairly sedentary (little or no exercise and desk job) |
1.2 |
|
Lightly active (light exercise or sports 1 to 3 days per week) |
1.375 |
|
Moderately active (moderate exercise or sports 3 to 5 days per week) |
1.55 |
|
Very active (hard exercise or sports 6 to 7 days per week) |
1.725 |
|
Extremely active (hard daily exercise or sports and physical job) |
1.9 |
In the past, humans had to work hard to find their food and sometimes came up empty-handed. To survive, the human body developed a mechanism for storing energy that can be used during times of low food intake. It packs energy-rich fat onto your hips, thighs, abdomen, and buttocks. So if you take in more Calories in a day than you need, the extra Calories are stored as fat in your adipose tissue. Every 3,500 extra Calories equals 1 pound of fat. And your body doesn’t give up extra potential energy easily! If you continue to take in more Calories than you use, you willgain weight because it’s much easier for your body to create fat than to use it.