Harper’s Illustrated Biochemistry, 29th Edition (2012)
SECTION I. Structures & Functions of Proteins & Enzymes
Chapter 3. Amino Acids & Peptides
Peter J. Kennelly, PhD & Victor W. Rodwell, PhD
OBJECTIVES
After studying this chapter, you should be able to:
![]() Name, and draw the structures of, the 20 amino acids present in proteins.
Name, and draw the structures of, the 20 amino acids present in proteins.
![]() Write the three- and one-letter designations for each of the common amino acids.
Write the three- and one-letter designations for each of the common amino acids.
![]() List the ionizable groups of the common amino acids and their pKa values.
List the ionizable groups of the common amino acids and their pKa values.
![]() Calculate the pH of an unbuffered aqueous solution of a polyfunctional amino acid and the change in pH that occurs following the addition of a given quantity of strong acid or alkali.
Calculate the pH of an unbuffered aqueous solution of a polyfunctional amino acid and the change in pH that occurs following the addition of a given quantity of strong acid or alkali.
![]() Define pI and indicate its relationship to the net charge on a polyfunctional electrolyte.
Define pI and indicate its relationship to the net charge on a polyfunctional electrolyte.
![]() Explain how pH, pKa and pI can be used to predict the mobility of a polyelectrolyte, such as an amino acid, in a direct-current electrical field.
Explain how pH, pKa and pI can be used to predict the mobility of a polyelectrolyte, such as an amino acid, in a direct-current electrical field.
![]() Describe the contribution of each type of R group of the common amino acids to their chemical properties.
Describe the contribution of each type of R group of the common amino acids to their chemical properties.
![]() Describe the directionality, nomenclature, and primary structure of peptides.
Describe the directionality, nomenclature, and primary structure of peptides.
![]() Identify the bond in a peptide that exhibits partial double-bond character and its conformational consequences in a peptide.
Identify the bond in a peptide that exhibits partial double-bond character and its conformational consequences in a peptide.
![]() Identify those bonds in the peptide backbone that are capable of free rotation and the Greek letters used to designate them.
Identify those bonds in the peptide backbone that are capable of free rotation and the Greek letters used to designate them.
BIOMEDICAL IMPORTANCE
In addition to providing the monomer units from which the long polypeptide chains of proteins are synthesized, the L-α-amino acids and their derivatives participate in cellular functions as diverse as nerve transmission and the biosynthesis of porphyrins, purines, pyrimidines, and urea. Short polymers of amino acids called peptides perform prominent roles in the neuroendocrine system as hormones, hormone-releasing factors, neuromodulators, or neurotransmitters. Humans and other higher animals lack the capability to synthesize 10 of the 20 common L-α-amino acids in amounts adequate to support infant growth or to maintain health in adults. Consequently, the human diet must contain adequate quantities of these nutritionally essential amino acids. While human proteins contain only L-α-amino acids, microorganisms make extensive use of D-α-amino acids. Bacillus subtilis, for example, secretes a mixture of D-methionine, D-tyrosine, D-leucine, and D-tryptophan to trigger biofilm disassembly, and Vibrio cholerae incorporates D-leucine and D-methionine into the peptide component of their peptidoglycan layer. Many bacteria elaborate peptides that contain both D- and L-α-amino acids, several of which possess therapeutic value, including the antibiotics bacitracin and gramicidin A and the antitumor agent bleomycin. Certain other microbial peptides are toxic. The cyanobacterial peptides microcystin and nodularin are lethal in large doses, while small quantities promote the formation of hepatic tumors.
PROPERTIES OF AMINO ACIDS
The Genetic Code Specifies 20 L-α-Amino Acids
Of the over 300 naturally occurring amino acids, 20 constitute the predominant monomer units of proteins. While a three-letter genetic code could potentially accommodate more than 20 amino acids, several amino acids are specified by multiple codons (see Table 37–1). Redundant usage limits the available codons to the 20 L-α-amino acids listed in Table 3–1. Both one- and three-letter abbreviations for each amino acid can be used to represent the amino acids in peptides and proteins (Table 3–1). Some proteins contain additional amino acids that arise by modification of an amino acid already present in a peptide. Examples include conversion of peptidyl proline and lysine to 4-hydroxyproline and 5-hydroxylysine; the conversion of peptidyl glutamate to γ-carboxyglutamate; and the methylation, formylation, acetylation, prenylation, and phosphorylation of certain aminoacyl residues. These modifications extend the biologic diversity of proteins by altering their solubility, stability, and interaction with other proteins.
TABLE 3–1 L-α-Amino Acids Present in Proteins

Selenocysteine, the 21st L-α-Amino Acid
Selenocysteine is an L-α-amino acid found in proteins from every domain of life. Humans contain approximately two dozen selenoproteins that include certain peroxidases and reductases, selenoprotein P which circulates in the plasma, and the iodothyronine deiodinases responsible for converting the prohormone thyroxine (T4) to the thyroid hormone 3,3′5-triiodothyronine (T3) (Chapter 41). As the name implies, a selenium atom replaces the sulfur of its structural analog, cysteine. The pK3 of selenocysteine, 5.2, is three units lower than that of cysteine. Unlike other unusual amino acids, selenocysteine is not the product of a posttranslational modification. Rather, it is inserted directly into a growing polypeptide during translation. Selenocysteine thus is commonly referred to as the “21st amino acid.” However, unlike other 20 genetically encoded amino acids, selenocysteine is specified by a much larger and more complex genetic element than the basic three-letter codon (see Chapter 27).
Only L-α-Amino Acids Occur in Proteins
With the sole exception of glycine, the α-carbon of every amino acid is chiral. Although some protein amino acids are dextrorotatory and some levorotatory, all share the absolute configuration of L-glyceraldehyde and thus are defined as L-α-amino acids. Several free L-α-amino acids fulfill important roles in metabolic processes. Examples include ornithine, citrulline, and argininosuccinate that participate in urea synthesis, tyrosine in formation of thyroid hormones, and glutamate in neurotransmitter biosynthesis. D-amino acids that occur naturally include free D-serine and D-aspartate in brain tissue, D-alanine and D-glutamate in the cell walls of gram-positive bacteria, and D-amino acids in certain peptides and antibiotics produced by bacteria, fungi, reptiles, and other nonmammalian species.
Amino Acids May Have Positive, Negative, or Zero Net Charge
Charged and uncharged forms of the ionizable—COOH and ![]() weak acid groups that exist in solution in protonic equilibrium:
weak acid groups that exist in solution in protonic equilibrium:
![]()
While both R—COOH and ![]() are weak acids, R—COOH is a far stronger acid than
are weak acids, R—COOH is a far stronger acid than ![]() . Thus, at physiologic pH (pH 7.4), carboxyl groups exist almost entirely as R—COO– and amino groups predominantly as
. Thus, at physiologic pH (pH 7.4), carboxyl groups exist almost entirely as R—COO– and amino groups predominantly as ![]() . Figure 3–1 illustrates the effect of pH on the charged state of aspartic acid.
. Figure 3–1 illustrates the effect of pH on the charged state of aspartic acid.

FIGURE 3–1 Protonic equilibria of aspartic acid.
Molecules that contain an equal number of ionizable groups of opposite charge and that therefore bear no net charge are termed zwitterions. Amino acids in blood and most tissues thus should be represented as in A, below.
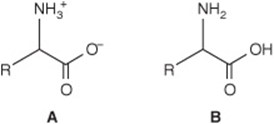
Structure B cannot exist in an aqueous solution because at any pH low enough to protonate the carboxyl group, the amino group would also be protonated. Similarly, at any pH sufficiently high for an uncharged amino group to predominate, a carboxyl group will be present as R—COO–. The uncharged representation B is, however, often used for reactions that do not involve protonic equilibria.
pKa Values Express the Strengths of Weak Acids
The acid strengths of weak acids are expressed as their pKa. For molecules with multiple dissociable protons, the pKa for each acidic group is designated by replacing the subscript “a” with a number (Table 3–1). The imidazole group of histidine and the guanidino group of arginine exist as resonance hybrids with positive charge distributed between both nitrogens (histidine) or all three nitrogens (arginine) (Figure 3–2). The net charge on an amino acid—the algebraic sum of all the positively and negatively charged groups present—depends upon the pKa values of its functional groups and on the pH of the surrounding medium. Altering the charge on amino acids and their derivatives by varying the pH facilitates the physical separation of amino acids, peptides, and proteins (see Chapter 4).
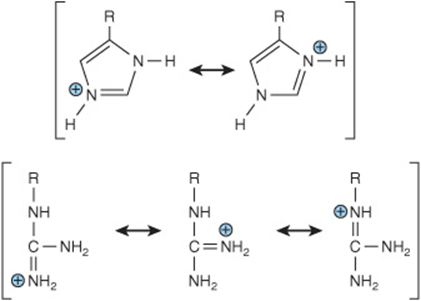
FIGURE 3–2 Resonance hybrids of the protonated forms of the R groups of histidine and arginine.
At its Isoelectric pH (pI), an Amino Acid Bears No Net Charge
Zwitterions are one example of an isoelectric species—the form of a molecule that has an equal number of positive and negative charges and thus is electrically neutral. The isoelectric pH, also called the pI, is the pH midway between pKa values for the ionizations on either side of the isoelectric species. For an amino acid such as alanine that has only two dissociating groups, there is no ambiguity. The first pKa (R—COOH) is 2.35 and the second ![]() is 9.69. The isoelectric pH (pI) of alanine thus is
is 9.69. The isoelectric pH (pI) of alanine thus is
![]()
For polyprotic acids, pI is also the pH midway between the pKa values on either side of the isoionic species. For example, the pI for aspartic acid is
![]()
For lysine, pI is calculated from
![]()
Similar considerations apply to all polyprotic acids (eg, proteins), regardless of the number of dissociating groups present. In the clinical laboratory, knowledge of the pI guides selection of conditions for electrophoretic separations. For example, electrophoresis at pH 7.0 will separate two molecules with pI values of 6.0 and 8.0, because at pH 7.0 the molecule with a pI of 6.0 will have a net positive charge, and that with a pI of 8.0 a net negative charge. Similar considerations apply to understanding chromatographic separations on ionic supports such as diethylaminoethyl (DEAE) cellulose (see Chapter 4).
pKa Values Vary with the Environment
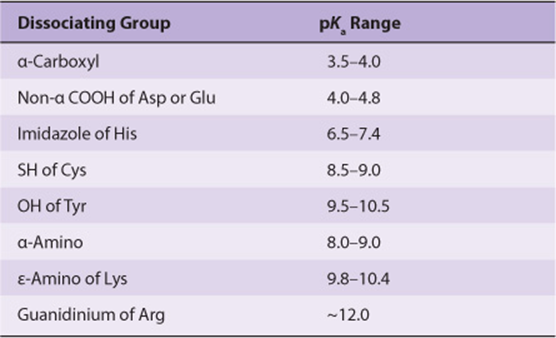
The environment of a dissociable group affects its pKa. The pKa values of the R groups of free amino acids in an aqueous solution (Table 3–1) thus provide only an approximate guide to the pKa values of the same amino acids when present in proteins. A polar environment favors the charged form (R—COO– or ![]() ), and a nonpolar environment favors the uncharged form (R—COOH or R—NH2). A nonpolar environment thus raises the pKa of a carboxyl group (making it a weaker acid) but lowers that of an amino group (making it a stronger acid). The presence of adjacent charged groups can reinforce or counteract solvent effects. The pKa of a functional group thus will depend upon its location within a given protein. Variations in pKa can encompass whole pH units (Table 3–2). pKa values that diverge from those listed by as much as 3 pH units are common at the active sites of enzymes. An extreme example, a buried aspartic acid of thioredoxin, has a pKa above 9—a shift of more than 6 pH units.
), and a nonpolar environment favors the uncharged form (R—COOH or R—NH2). A nonpolar environment thus raises the pKa of a carboxyl group (making it a weaker acid) but lowers that of an amino group (making it a stronger acid). The presence of adjacent charged groups can reinforce or counteract solvent effects. The pKa of a functional group thus will depend upon its location within a given protein. Variations in pKa can encompass whole pH units (Table 3–2). pKa values that diverge from those listed by as much as 3 pH units are common at the active sites of enzymes. An extreme example, a buried aspartic acid of thioredoxin, has a pKa above 9—a shift of more than 6 pH units.
TABLE 3–2 Typical Range of pKa values for Ionizable Groups in Proteins
The Solubility of Amino Acids Reflects their Ionic Character
The charges conferred by the dissociable functional groups of amino acids ensure that they are readily solvated by—and thus soluble in—polar solvents such as water and ethanol but insoluble in nonpolar solvents such as benzene, hexane, or ether.
Amino acids do not absorb visible light and thus are colorless. However, tyrosine, phenylalanine, and especially tryptophan absorb high-wavelength (250–290 nm) ultraviolet light. Because it absorbs ultraviolet light about ten times more efficiently than phenylalanine or tyrosine, tryptophan makes the major contribution to the ability of most proteins to absorb light in the region of 280 nm.
THE α–R GROUPS DETERMINE THE PROPERTIES OF AMINO ACIDS
Since glycine, the smallest amino acid, can be accommodated in places inaccessible to other amino acids, it often occurs where peptides bend sharply. The hydrophobic R groups of alanine, valine, leucine, and isoleucine and the aromatic R groups of phenylalanine, tyrosine, and tryptophan typically occur primarily in the interior of cytosolic proteins. The charged R groups of basic and acidic amino acids stabilize specific protein conformations via ionic interactions or salt bridges. These interactions also function in “charge relay” systems during enzymatic catalysis and electron transport in respiring mitochondria. Histidine plays unique roles in enzymatic catalysis. The pKa of its imidazole proton permits histidine to function at neutral pH as either a base or an acid catalyst without the need for any environmentally induced shift. The primary alcohol group of serine and the primary thioalcohol (—SH) group of cysteine are excellent nucleophiles, and can function as such during enzymatic catalysis. However, the secondary alcohol group of threonine, while a good nucleophile, is not known to fulfill an analogous role in catalysis. The—OH groups of serine, tyrosine, and threonine also participate in regulation of the activity of enzymes whose catalytic activity depends on the phosphorylation state of these residues.
FUNCTIONAL GROUPS DICTATE THE CHEMICAL REACTIONS OF AMINO ACIDS
Each functional group of an amino acid exhibits all of its characteristic chemical reactions. For carboxylic acid groups, these reactions include the formation of esters, amides, and acid anhydrides; for amino groups, acylation, amidation, and esterification; and for—OH and—SH groups, oxidation, and esterification. The most important reaction of amino acids is the formation of a peptide bond (shaded).
Amino Acid Sequence Determines Primary Structure
The number and order of all the amino acid residues in a polypeptide constitute its primary structure. Amino acids present in peptides are called aminoacyl residues and are named by replacing the -ate or -ine suffixes of free amino acids with -yl (eg, alanyl, aspartyl, tyrosyl). Peptides are then named as derivatives of the carboxy terminal aminoacyl residue. For example, Lys-Leu-Tyr-Gln is called lysyl-leucyl-tyrosyl-glutamine. The -ine ending on glutamine indicates that its α-carboxyl group is not involved in peptide bond formation.
Peptide Structures Are Easy to Draw
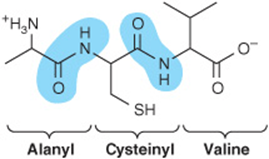
Prefixes such as tri- or octa- denote peptides with three or eight residues, respectively. By convention, peptides are written with the residue that bears the free α-amino group on the left. To draw a peptide, use a zigzag to represent the main chain or backbone. Add the main chain atoms, which occur in the repeating order: α-nitrogen, α-carbon, and carbonyl carbon. Now add a hydrogen atom to each α-carbon and to each peptide nitrogen, and an oxygen atom to the carbonyl carbon. Finally, add the appropriate R groups (shaded) to each α-carbon atom.
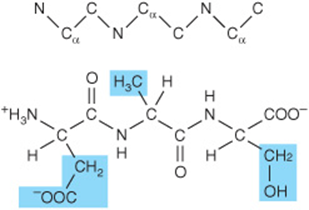
Three-letter abbreviations linked by straight lines represent an unambiguous primary structure. Lines are omitted for single-letter abbreviations.
![]()
Some Peptides Contain Unusual Amino Acids
In mammals, peptide hormones typically contain only the 20 genetically encoded α-amino acids linked by standard peptide bonds. Other peptides may, however, contain nonprotein amino acids, derivatives of the protein amino acids, or amino acids linked by an atypical peptide bond. For example, the amino terminal glutamate of glutathione, a tripeptide that participates in protein folding and in the metabolism of xenobiotics (Chapter 53), is linked to cysteine by a non-α peptide bond (Figure 3–3). The amino terminal glutamate of thyrotropin-releasing hormone (TRH) is cyclized to pyroglutamic acid, and the carboxyl group of the carboxyl terminal prolyl residue is amidated. The nonprotein amino acids D-phenylalanine and ornithine are present in the cyclic peptide antibiotics tyrocidin and gramicidin S, while the heptapeptide opioids dermorphin and deltophorin in the skin of South American tree frogs contain D-tyrosine and D-alanine.
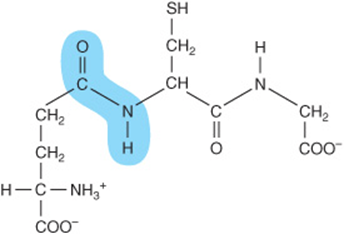
FIGURE 3–3 Glutathione (γ-glutamyl-cysteinyl-glycine). Note the non-α peptide bond that links Glu to Cys.
Peptides are Polyelectrolytes
The peptide bond is uncharged at any pH of physiologic interest. Formation of peptides from amino acids is therefore accompanied by a net loss of one positive and one negative charge per peptide bond formed. Peptides nevertheless are charged at physiologic pH owing to their terminal carboxyl and amino groups and, where present, their acidic or basic R groups. As for amino acids, the net charge on a peptide depends on the pH of its environment and on the pKa values of its dissociating groups.
The Peptide Bond Has a Partial Double-Bond Character
Although peptides are written as if a single bond linked the α-carboxyl and α-nitrogen atoms, this bond in fact exhibits a partial double-bond character:
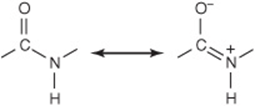
There thus is no freedom of rotation about the bond that connects the carbonyl carbon and the nitrogen of a peptide bond. Consequently, the O, C, N, and H atoms of a peptide bond are coplanar. The imposed semirigidity of the peptide bond has important consequences for the manner in which peptides and proteins fold to generate higher order of structure. Encircling brown arrows (Figure 3–4) indicate free rotation about the remaining bonds of the polypeptide backbone.
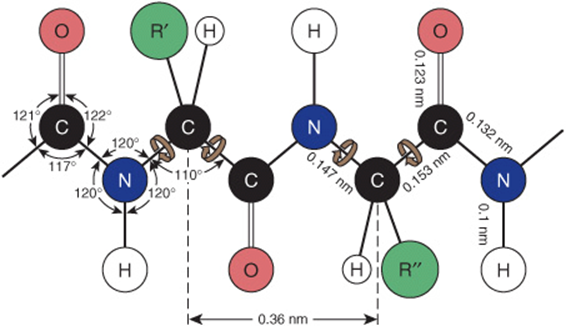
FIGURE 3–4 Dimensions of a fully extended polypeptide chain. The four atoms of the peptide bond are coplanar. Free rotation can occur about the bonds that connect the α-carbon with the α-nitrogen and with the α-carbonyl carbon (brown arrows). The extended polypeptide chain is thus a semirigid structure with two-thirds of the atoms of the backbone held in a fixed planar relationship one to another. The distance between adjacent α-carbon atoms is 0.36 nm (3.6 Å). The interatomic distances and bond angles, which are not equivalent, are also shown. (Redrawn and reproduced, with permission, from Pauling L, Corey LP, Branson HR: The structure of proteins: Two hydrogen-bonded helical configurations of the polypeptide chain. Proc Natl Acad Sci USA 1951;37:205.)
Noncovalent Forces Constrain Peptide Conformations
Folding of a peptide probably occurs coincident with its biosynthesis (see Chapter 37). The physiologically active conformation reflects the collective contributions of the amino acid sequence, steric hindrance, and noncovalent interactions (eg, hydrogen bonding, hydrophobic interactions) between residues. Common conformations include α-helices and β-pleated sheets (see Chapter 5).
ANALYSIS OF THE AMINO ACID CONTENT OF BIOLOGIC MATERIALS
To determine the identity of each amino acid present in a protein, it is first treated with hot hydrochloric acid to hydrolyze the peptide bonds. There are several methods for separation and identification of amino acids derived from a protein hydrolysate or from urine or other biologic fluids. One approach is to react the amino acids with 6-amino-N-hydroxysuccinimidyl carbamate to form fluorescent derivatives that can be separated by high-pressure liquid chromatography (see Chapter 4). An alternative approach, which requires only minimal equipment, employs partition chromatography on a solid support, typically a sheet of filter paper (paper chromatography) or a thin layer of powdered cellulose or silica gel on an inert support (thin-layer chromatography, or TLC). The amino acids present are resolved by a mobile phase that contains a mixture of miscible polar and nonpolar components (eg, n-butanol, formic acid, and water). As the mobile phase moves up the sheet, its polar components associate with the polar groups of the support. The solvent therefore becomes progressively less polar as it migrates up the sheet. The amino acids therefore partition between a polar stationary phase and a less polar mobile phase (“partition chromatography”). Nonpolar amino acids (eg, Leu, Ile) migrate the farthest as they spend the greatest proportion of their time in the mobile phase. Polar amino acids (eg, Glu, Lys) travel the least distance from the origin as they spend a high proportion of their time in the stationary phase consisting of a layer of polar solvent molecules immobilized by their association with the cellulose or silica support. Following removal of the solvent by air drying, amino acids are visualized using ninhydrin, which forms purple products with α-amino acids, but a yellow adduct with proline and hydroxyproline.
SUMMARY
![]() Both D-amino acids and non-α-amino acids occur in nature, but only L-α-amino acids are present in proteins.
Both D-amino acids and non-α-amino acids occur in nature, but only L-α-amino acids are present in proteins.
![]() All amino acids possess at least two weakly acidic functional groups,
All amino acids possess at least two weakly acidic functional groups, ![]() and R—COOH. Many also possess additional weakly acidic functional groups such as—OH,—SH, guanidino, or imidazole moieties.
and R—COOH. Many also possess additional weakly acidic functional groups such as—OH,—SH, guanidino, or imidazole moieties.
![]() The pKa values of all functional groups of an amino acid dictate its net charge at a given pH. pI is the pH at which an amino acid bears no net charge and thus does not move in a direct current electrical field.
The pKa values of all functional groups of an amino acid dictate its net charge at a given pH. pI is the pH at which an amino acid bears no net charge and thus does not move in a direct current electrical field.
![]() Of the biochemical reactions of amino acids, the most important is the formation of peptide bonds.
Of the biochemical reactions of amino acids, the most important is the formation of peptide bonds.
![]() The R groups of amino acids determine their unique biochemical functions. Amino acids are classified as basic, acidic, aromatic, aliphatic, or sulfur containing based on the properties of their R groups.
The R groups of amino acids determine their unique biochemical functions. Amino acids are classified as basic, acidic, aromatic, aliphatic, or sulfur containing based on the properties of their R groups.
![]() Peptides are named for the number of amino acid residues present, and as derivatives of the carboxyl terminal residue. The primary structure of a peptide is its amino acid sequence, starting from the amino-terminal residue.
Peptides are named for the number of amino acid residues present, and as derivatives of the carboxyl terminal residue. The primary structure of a peptide is its amino acid sequence, starting from the amino-terminal residue.
![]() The partial double-bond character of the bond that links the carbonyl carbon and the nitrogen of a peptide renders four atoms of the peptide bond coplanar and restricts the number of possible peptide conformations.
The partial double-bond character of the bond that links the carbonyl carbon and the nitrogen of a peptide renders four atoms of the peptide bond coplanar and restricts the number of possible peptide conformations.
REFERENCES
Doolittle RF: Reconstructing history with amino acid sequences. Protein Sci 1992;1:191.
Gladyschev VN, Hatfield DL: Selenocysteine-containing proteins in mammals. J Biomed Sci 1999;6:151.
Kolodkin-Gal I: D-Amino acids trigger biofilm disassembly. Science 2010;328:627.
Kreil G: D-Amino acids in animal peptides. Annu Rev Biochem 1997;66:337.
Nokihara K, Gerhardt J: Development of an improved automated gas-chromatographic chiral analysis system: application to nonnatural amino acids and natural protein hydrolysates. Chirality 2001;13:431.
Papp LV: From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxidants Redox Signal 2007;9:775.
Sanger F: Sequences, sequences, and sequences. Annu Rev Biochem 1988;57:1.
Stadtman TC: Selenocysteine. Annu Rev Biochem 1996;65:83.
Wilson NA, Barbar E, Fuchs JA, et al: Aspartic acid 26 in reduced Escherichia coli thioredoxin has a pKa greater than 9. Biochemistry 1995;34:8931.