CHEMISTRY THE CENTRAL SCIENCE
24 THE CHEMISTRY OF LIFE: ORGANIC AND BIOLOGICAL CHEMISTRY
24.8 CARBOHYDRATES
Carbohydrates are an important class of naturally occurring substances found in both plant and animal matter. The name carbohydrate (“hydrate of carbon”) comes from the empirical formulas for most substances in this class, which can be written as Cx(H2O)y. For example, glucose, the most abundant carbohydrate, has the molecular formula C6H12O6, or C6(H2O)6. Carbohydrates are not really hydrates of carbon; rather, they are polyhydroxy aldehydes and ketones. Glucose, for example, is a six-carbon aldehyde sugar, whereas fructose, the sugar that occurs widely in fruit, is a six-carbon ketone sugar (![]() FIGURE 24.21).
FIGURE 24.21).
The glucose molecule, having both alcohol and aldehyde functional groups and a reasonably long and flexible backbone, can form a six-member-ring structure, as shown in ![]() FIGURE 24.22. In fact, in an aqueous solution only a small percentage of the glucose molecules are in the open-chain form. Although the ring is often drawn as if it were planar, the molecules are actually nonplanar because of the tetrahedral bond angles around the C and O atoms of the ring.
FIGURE 24.22. In fact, in an aqueous solution only a small percentage of the glucose molecules are in the open-chain form. Although the ring is often drawn as if it were planar, the molecules are actually nonplanar because of the tetrahedral bond angles around the C and O atoms of the ring.
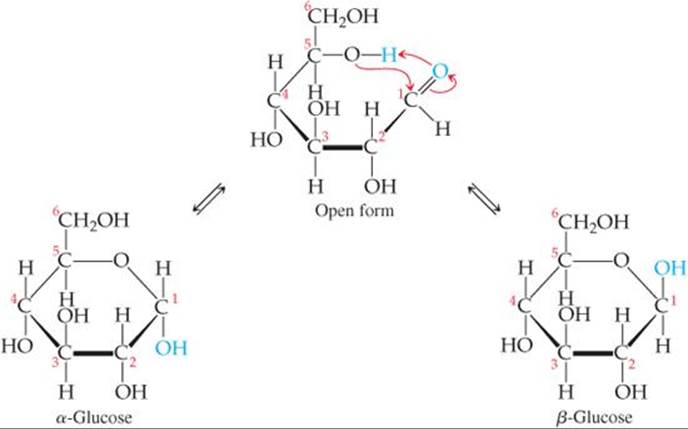
![]() FIGURE 24.22 Cyclic glucose has an α form and a β form.
FIGURE 24.22 Cyclic glucose has an α form and a β form.
Figure 24.22 shows that the ring structure of glucose can have two relative orientations. In the α form the OH group on C1 and the CH2OH group on C5 point in opposite directions, and in the β form they point in the same direction. Although the difference between the α and β forms might seem small, it has enormous biological consequences, including the vast difference in properties between starch and cellulose.
Fructose can cyclize to form either five- or six-member rings. The five-member ring forms when the C5 OH group reacts with the C2 carbonyl group:
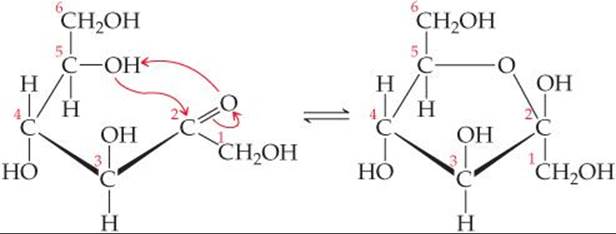
The six-member ring results from the reaction between the C6 OH group and the C2 carbonyl group.
SAMPLE EXERCISE 24.8 Identifying Chiral Centers
How many chiral carbon atoms are there in the open-chain form of glucose (Figure 24.21)?
SOLUTION
Analyze We are given the structure of glucose and asked to determine the number of chiral carbons in the molecule.
Plan A chiral carbon has four different groups attached (Section 24.5). We need to identify those carbon atoms in glucose.
Solve Carbons 2, 3, 4, and 5 each have four different groups attached to them:
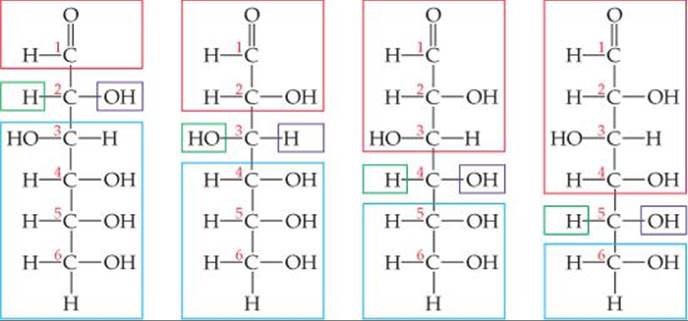
Thus, there are four chiral carbon atoms in the glucose molecule.
PRACTICE EXERCISE
How many chiral carbon atoms are there in the open-chain form of fructose (Figure 24.21)?
Answer: three
Disaccharides
Both glucose and fructose are examples of monosaccharides, simple sugars that cannot be broken into smaller molecules by hydrolysis with aqueous acids. Two monosaccharide units can be linked together by a condensation reaction to form a disaccharide. The structures of two common disaccharides, sucrose (table sugar) and lactose (milk sugar), are shown in ![]() FIGURE 24.23.
FIGURE 24.23.
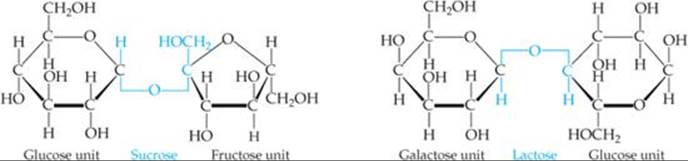
![]() FIGURE 24.23 Two disaccharides.
FIGURE 24.23 Two disaccharides.
The word sugar makes us think of sweetness. All sugars are sweet, but they differ in the degree of sweetness we perceive when we taste them. Sucrose is about six times sweeter than lactose, slightly sweeter than glucose, but only about half as sweet as fructose. Disaccharides can be reacted with water (hydrolyzed) in the presence of an acid catalyst to form monosaccharides. When sucrose is hydrolyzed, the mixture of glucose and fructose that forms, called invert sugar,* is sweeter to the taste than the original sucrose. The sweet syrup present in canned fruits and candies is largely invert sugar formed from hydrolysis of added sucrose.
Polysaccharides
Polysaccharides are made up of many monosaccharide units joined together. The most important polysaccharides are starch, glycogen, and cellulose, all three of which are formed from repeating glucose units.
Starch is not a pure substance. The term refers to a group of polysaccharides found in plants. Starches serve as a major method of food storage in plant seeds and tubers. Corn, potatoes, wheat, and rice all contain substantial amounts of starch. These plant products serve as major sources of needed food energy for humans. Enzymes in the digestive system catalyze the hydrolysis of starch to glucose.
Some starch molecules are unbranched chains, whereas others are branched. ![]() FIGURE 24.24 (a) illustrates an unbranched starch structure. Notice, in particular, that the glucose units are in the α form with the bridging oxygen atoms pointing in one direction and the CH2OH groups pointing in the opposite direction
FIGURE 24.24 (a) illustrates an unbranched starch structure. Notice, in particular, that the glucose units are in the α form with the bridging oxygen atoms pointing in one direction and the CH2OH groups pointing in the opposite direction
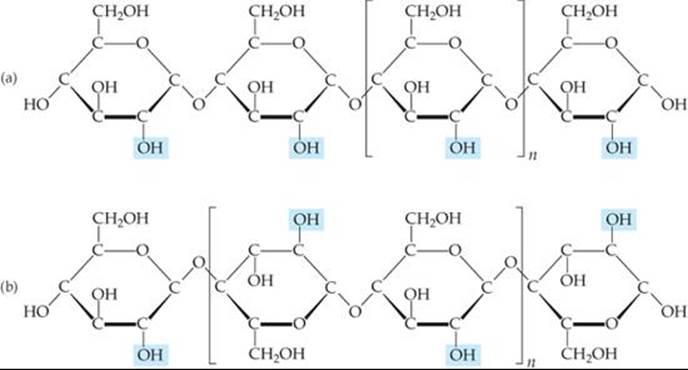
![]() FIGURE 24.24 Structures of (a) starch and (b) cellulose.
FIGURE 24.24 Structures of (a) starch and (b) cellulose.
Glycogen is a starchlike substance synthesized in the animal body. Glycogen molecules vary in molecular weight from about 5000 to more than 5 million amu. Glycogen acts as a kind of energy bank in the body. It is concentrated in the muscles and liver. In muscles it serves as an immediate source of energy; in the liver it serves as a storage place for glucose and helps to maintain a constant glucose level in the blood.
Cellulose [Figure 24.24(b)] forms the major structural unit of plants. Wood is about 50% cellulose; cotton fibers are almost entirely cellulose. Cellulose consists of an un-branched chain of glucose units, with molecular weights averaging more than 500,000 amu. At first glance this structure looks very similar to that of starch. In cellulose, however, the glucose units are in the β form with each bridging oxygen atom pointing in the same direction as the CH2OH group in the ring to its left.
Because the individual glucose units have different relationships to one another in starch and cellulose, enzymes that readily hydrolyze starches do not hydrolyze cellulose. Thus, you might eat a pound of cellulose and receive no caloric value from it even though the heat of combustion per unit mass is essentially the same for both cellulose and starch. A pound of starch, in contrast, would represent a substantial caloric intake. The difference is that the starch is hydrolyzed to glucose, which is eventually oxidized with release of energy. However, enzymes in the body do not readily hydrolyze cellulose, so it passes through the digestive system relatively unchanged. Many bacteria contain enzymes, called cellulases, that hydrolyze cellulose. These bacteria are present in the digestive systems of grazing animals, such as cattle, that use cellulose for food.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Which type of linkage, α or β, would you expect to join the sugar molecules of glycogen?