CHEMICAL BIOLOGY
Natural Products in Plants, Chemical Diversity of
A. Leslie Gunatilaka, Southwest Center for Natural Products Research, University of Arizona, Tucson, Arizona
doi: 10.1002/9780470048672.wecb163
Plants contain numerous natural products (secondary metabolites) that may not participate directly in their growth and development but play an important role in ecological interactions with other organisms. Despite immense chemical diversity, which originates from simple carbohydrates produced because of photosynthesis, plant natural products are formed from only a few biosynthetic building blocks that consist of acetate, mevalonate, and shikimate. These basic building blocks undergo a variety of biosynthetic transformations and combinations that lead to numerous classes of plant natural products including, but not limited to, carbohydrates, fatty acids and their esters, aromatic polyketides (phenols and quinones), terpenoids and steroids, phenyl propanoids (lignans and lignin, coumarins, flavonoids, and isoflavonoids), and alkaloids. Summarized in this article are representative members of these important classes of plant natural products with special emphasis on their chemical diversity. The article concludes with a brief discussion on recent methods for the maximization of chemical diversity and the production of natural products from plants.
The number of different plant species on the surface of the earth has been estimated to be over 250,000 (1, 2), and only a fraction of these have been investigated for their constituent natural products (3). Plants are known to produce over 100,000 natural products (4). However, according to Verpoorte (5) “extrapolations of the number of species studied and the number of compounds known suggests that, from all plant species, at least a million different compounds could be isolated”. The vast majority of these compounds, commonly referred to as secondary metabolites, does not seem to participate directly in the growth and development of plants (6). In their natural environments, plants coexist and interact with other organisms in a variety of ecosystems (7), and the possible roles that these natural products play in plants, especially in the context of ecological interactions, are being speculated about, appreciated, and debated (8, 9). Although the functional role that secondary metabolites play in the producing organism is a matter of controversy (10, 11), the chemical diversity of plant natural products is well recognized, and it has been suggested that the chemical diversity of plant natural products is far greater than their functional diversity (9, 10, 12, 13).
Two models exist to explain the abundant chemical diversity of plant natural products. In the first model, secondary metabolites produced by plants are believed to be involved in physiological responses during the interactions with their biotic and abiotic environments, especially as elements of their defense arsenals. In this model, the diversity of compounds produced by plants is explained by considering the great diversity of plant life strategies and the vast number of accompanying defense strategies (14-16). The second model is an evolutionary model that makes the assumption that potent biological activity is a rare property for any natural product to possess and therefore is of no value to the producer organism (8). This model, based on the Screening Hypothesis of Jones and Firn (10, 17, 18), suggests that organisms that make and “screen” many chemicals will have an increased likelihood of enhanced fitness simply because the greater the chemical diversity the greater the chances of producing the rare metabolites with useful and potent biological activities.
Origin of Natural Products in Plants
It is intriguing that despite the immense chemical diversity exhibited by them, plant natural products are derived from only a few building blocks: acetate (which contains two carbon atoms), mevalonate (which contains five carbon atoms), and shikimate (which contains nine carbon atoms). These building blocks are derived in turn from simple carbohydrates produced because of the light-catalyzed reduction of atmospheric carbon dioxide by higher (green) plants during photosynthesis. The products formed by the condensation of the above building blocks (small biosynthetic units) are additionally elaborated (“tailored” or “decorated”) by numerous enzyme-catalyzed reactions such as cyclization, elimination, rearrangement, reduction, oxidation, methylation, and so forth. Chemical diversity that results from these “decoration” reactions will be considered under each biosynthetic class of plant natural products.
Carbohydrates
Although not as structurally diverse as other classes of natural products, carbohydrates are among the most abundant chemical constituents of plants. All animals and most microorganisms depend on plant-derived carbohydrates for their nourishment and survival. Simple carbohydrates (aldoses and ketoses), the first formed products of photosynthesis, are used by plants to make their food reserves, as starter units for the synthesis of plant secondary metabolites, and to make sugar derivatives (glycosides) of products of secondary metabolism. Plant carbohydrates consist of monosaccharides (pentoses and hexoses), disaccharides, oligosaccharides, and polysaccharides. Monosaccharides of plant origin include the stereoisomeric forms of hexose sugars β-D-glucose (A1), β-D-galactose (A2), β-D-mannose (A3), α-L-rhamnose (A4), β-D-xylose (A5), α-L-arabinose (A6), β-D-ribose (A7), and β-D-fructose (A8) (Fig. 1). L-ascorbic acid (A9), commonly known as vitamin C and occurring in most fresh fruits and vegetables, is a monosaccharide derived from D-glucose. As the name implies, disaccharides are formed from two monosaccharide units and contain a C-O-C link. Among the common plant dissacharides, maltose (A10) and lactose (A11) contain respectively C(1α)-O-C(4) and C(1β) -O-C(4) links formed between two D-glucose (A1) units, whereas sucrose (A12) contains the C(1α)-O-C(2P) link formed between D-glucose (A1) and D-fructose (A8) units (Fig. 1). Polysaccharides that are polymeric monosaccharides perform two major functional roles, namely, the food reserves and structural elements in plants. Amylose (A13), an example of a storage polysaccharide, is a linear polymer that contains 1000-2000 C(1α)-C(4) linked glucopyranose units. Cellulose is an example of a structural polysaccharide composed of a linear chain of ca. 8000 residues of C(1β)-C(4) linked glucopyranose units. It is noteworthy that cellulose, the main constituent in plant cell walls, is the most abundant organic material on earth.

Figure 1. Chemical diversity of plant carbohydrates.
Products of Acetate Pathway
The two-carbon precursor, acetyl coenzyme A (acetyl-CoA), is the initial substrate for synthesis of the carbon backbone of plant polyketides. As the name implies, polyketides are naturally occurring polymers of ketene (CH2CO) and contain alternating carbonyl and methylene groups derived from the acetate pathway. Polyketides and their derivatives are ubiquitous and are found in all organisms known to produce secondary metabolites. Because of their immense structural diversity, a unified classification of polyketides has yet to emerge (19). Plant polyketides are represented by two major classes of metabolites: fatty acids and aromatic compounds.
Fatty acids and their esters
Plants contain both saturated and unsaturated fatty acids mostly as esters of the trihydroxy alcohol, glycerol. As they are derived from the linear combination of acetate (C2) units, common fatty acids possess an even number of carbon atoms and contain a straight chain. Thus, fatty acids that contain an odd number of carbons are rare in nature. Over 300 fatty acids belonging to 18 structural classes occur in plants (20). Among these, the more common are saturated fatty acids that contain 16 or 18 carbon atoms such as lauric acid, myristic acid, palmitic acid, and stearic acid, and the unsaturated analogs of stearic acid, namely, oleic acid and linoleic acid. Oils produced by many plants constitute glycerol esters of both saturated and unsaturated fatty acids. The other classes of fatty acids are defined by the number and arrangement of double or triple bonds and various other functional groups.
Aromatic polyketides in plants
Aromatic natural products of polyketide origin are less prevalent in plants compared with microorganisms. The majority of the plant constituents that contain aromatic structures are known to arise from the shikimate pathway (see below). Unlike those derived from the shikimate pathway, aromatic products of the polyketide pathway invariably contain a meta oxygenation pattern because of their origin from the cyclization of polyketides. Phenolic compounds such as chrysophanol-anthrone (B1), and emodin-anthrone (B2), and the anthraquinones, aloe-emodin (B3) and emodin (B4) (Fig. 2), are products of the polyketide pathway and are found to occur in some plants of the genera Cassia (Leguminosae) (21), Rhamnus (Rhamnaceae) (22), and Aloe (Liliaceae) (23). The dimer of emodin-anthrone (B2), namely hypericin, (B5) is a constituent of the antidepressant herbal supplement, St. John’s wort (Hypericumperforatum, Hypericaceae) (24).

Figure 2. Examples of some aromatic polyketides in plants.
Products of Mevalonate Pathway
Mevalonic acid, a six-carbon building block, is made up from three molecules of the most basic two-carbon precursor, acetyl-CoA. The mevalonate pathway, which involves the intermediary of mevalonic acid, directs acetate into a series of natural products different from those derived directly from the acetate pathway and includes terpenoids and steroids. Terpenoids constitute the most chemically diverse and one of the largest groups of plant natural products, and therefore a detailed discussion on this group of natural products is warranted.
Most terpenoids are derived from mevalonic acid (MVA) through the universal precursor isopentenyl diphosphate (IPP) and its allylic isomer dimethylallyl diphosphate (DMAPP). Thus, the vast majority of terpenoids contain the basic structural residue 2-methylbutane, often less precisely referred to as isoprene units. These C5 hemiterpene units combine with each other in a variety of ways leading to mono- (Cio), sesqui- (C15), di- (C20), sester- (C25), tri- (C30), tetra- (C40), and poly- (C5n (n = >8)) terpenes. The primary products of condensation undergo more elaboration (reduction, oxidation, derivatization, etc.) and “decorations” that lead to terpenoid hydrocarbons, alcohols and their glycosides, ethers, aldehydes, ketones, and carboxylic acids and their esters, which makes terpenoids the most diverse class of plant natural products. It is noteworthy that over 40,000 different terpenoids have been isolated and characterized from natural sources including plants (25, 26). Terpenoids also represent a functionally diverse class of natural products. Although all the biological, ecological, and pharmacological functions of terpenoids are yet to be fully understood, they are known to have a variety of functions in the plant kingdom and in human health and nutrition. Many plants are known to produce volatile terpenes for the purpose of attracting specific insects for pollination or to keep away herbivorous animals; some plants produce toxic or bitter-tasting terpenes known as antifeedants to protect them from being eaten by animals. Most importantly, terpenoids also play functional roles in plants as growth regulators (phytohormones) and signaling compounds (sociohormones). Some important pharmacologically active terpenoids include the sesquiterpenoid artemisinin with antimalarial activity (27), the anticancer diterpenoid paclitaxel (Taxol®; Mead Johnson, Princeton, NJ) (28), and the terpenoid indole alkaloids vincristine and vinblastine with anticancer activity (29, 30).
Hemiterpenoids
Compared with other terpenoids, only a few true hemiter- penes are found in nature, and over 90 of these occur as glycosides (31). The most noteworthy example is isoprene (2-methylbut-2-ene), a volatile hemiterpenoid released by many trees. Other natural hemiterpenes include prenol (3-methyl-2-buten-1-ol) in flowers of Cananga odorata (Annonaceae) and hops (Hamulus lupulus, Cannabaceae). Its isomer, (S)-(-)-3-methyl-3-buten-2-ol is a constituent of the essential oils of grapefruit, hops, and oranges. Another hemiterpenoid, 4-methoxy-2-methyl-2-butanthiol, is responsible for the characteristic flavor of blackcurrant (Ribes nigrum, Saxifragaceae). Numerous plant natural products with ester moieties contain hemiterpenoid-derived carboxylic acid components such as 3-methyl-2-butenoic acid and its isomers, angelic and tiglic acids, as well as its saturated analog, isovaleric acid. The immediate biosynthetic precursors of hemiterpenoids, IPP and DMAPP, are often used by plants as alkylating agents during the formation of some natural products (meroterpenoids) of mixed biosynthetic origin.
Monoterpenoids
Monoterpenoids are responsible for fragrances and flavors of many plants and thus their products are used in perfumery and as spices. To date over 1,500 monoterpenoids are known, and these constitute acyclic, monocyclic, and bicyclic monoterpenoids (32), which occur in nature as hydrocarbons, alcohols, aldehydes, and carboxylic acids and their esters. Several acyclic monoterpenoid hydrocarbons are known, and these include trienes such as β-myrcene (C1), α-myrcene (C2), (Z)-α-ocimene (C3), (E)-α-ocimene (C4), (Z)-β-ocimene (C5), and (E)-β-ocimene (C6). β-Myrcene and β-ocimene are constituents of basil (Ocimum basilicum, Labiatae) and bay (Pimenta acris, Myrtaceae), pettitgrain (Citrus vulgaris, Rutaceae) leaves, strobiles of hops (Humulus lupulus, Cannabaceae), and several other essential oils. Unsaturated acyclic monoterpene alcohol constituents of plants and their derived aldehydes play a significant role in the perfume industry. Some common acyclic monoterpene alcohols and aldehydes include geraniol (C7), linalool (C8) (a constituent of coriander oil), (R)-3,7-dimethyloctanol (C9) (of geranium oil), citranellol (C10) (of rose oil), geranial (C11) (of lemon oil), and citranellal (C12) (of citronella oil) (Fig. 3).

Figure 3. Structural diversity of acyclic monoterpenes.
Monoterpene precursors undergo a variety of cyclization and rearrangement reactions leading to diverse monocyclic and bicyclic monoterpenoids that consist of hydrocarbons, alcohols, and ketones. Compared with cyclopentane and cyclohexane analogs, cyclopropane and cyclobutane monoterpenoids that contain irregular monoterpene carbon skeletons are rare in nature. (+)-7rans-chrysanthemic acid (D1) and (+)-trans-pyrethric acid (D2) esters, which are known to occur in flower heads of Chrysanthemum cinerariaefolium (Compositae), are two important examples of cyclopropane monoterpenoids. Noteworthy examples of cyclobutane monoterpenoids are (1S ,2S)-fragranol (D3), which occurs in the roots of Artemisia fragrans (Asteraceae), and junionone (D4), which occurs in the fruits of the juniper tree (Juniperus communis, Cupressaceae) (Fig. 4).

Figure 4. Chemical diversity of monocyclic cyclopropane, cyclobutane, and cyclopentane monoterpenoids in plants.
To date about 200 cyclopentane monoterpenoids are known (32), and the majority of these in plants occur as iridoids and seco-iridoids that contain the iridane carbon skeleton fused to a six-membered oxygen heterocycle. The simplest iridoid, (+)-nepetalactone (D5), is a constituent of the volatile oil of Nepeta cataria (Labiatae), which is known to be a powerful cat attractant and stimulant. Other well-known plant-derived iridoids consist of a diverse array of valepotriates known to occur in the popular herbal supplement valerian (Valeriana officinalis, Valerianaceae). Most of these valepotriates, including (+)-valtrate (D6), the constituent responsible for the tranquilizing properties of the valerian, contain several hydroxyl groups esterified with the C5 hemiterpene isovaleric acid. Glucosides of iridoids also occur as plant constituents. Important examples are (-)-asperuloside (D7) with insect antifeedant activity in Asperula odorata (Galium odoratum, Rubiaceae) and many other plants and (—)-loganin (D8) from the fruits of Strychnos nux vomica (Loganiaceae). Although not as prevalent as iridoids, the seco-iridoids, (—)-oleuropin (D9), (+)-jasmolactone A (D10), and (—)-secologanin (D11) (Fig. 4), have been isolated from many parts of the olive tree (Olea europaea, Oleaceae), Jasminium multiflorum (Oleaceae) and Strychnos nux vomica (Loganiaceae), respectively.
Cyclohexane monoterpenes are a chemically diverse group of monoterpenoids that occur in the plant kingdom mainly as hydrocarbons, alcohols, ketones, aromatic hydrocarbons, and phenols (Fig. 5). The saturated hydrocarbon trans-p-menthane (E1) is a constituent of the oil of turpentine and the resin of pine (Pinaceae) trees. Its unsaturated analogs, namely (R)-(+)-limonene (E2) [present in oil of orange (Citrus aurantium) and mandarin (Citrus reticulata, Rutaceae) peel oil]; α-terpinene (E3) and terpinolene (E4) in some Citrus, Juniperus, Mentha and Pinus species; (R)-(-)-α-phellandrene (E5) in Eucalyptus phellandra (Myrtaceae); and (S )-(+)-β-phellandrene (E6) in water fennel (Phellandrium aquaticum, Umbelliferae), are components of many plant volatile oils. The rich chemical diversity of cyclohexane monoterpene alcohols is apparent from the natural occurrence of all four pairs of p-menthan-3-ol enantiomers, for example, (—)-menthol (E7) [a major component of peppermint (Mentha piperita, Labiatae) oil], (+)-neomenthol (E8) [a constituent of Japanese peppermint (Mentha arvensis) oil], and (—)-neoisomenthol (E9) in geranium (Pelargonium roseum, Geraniaceae) oil. The unsaturated versions of p-menthol, namely p-menthenol, exhibit extensive regioisomerism and are represented by (—)-pulegol (E10) (a constituent of several peppermint (Mentha gentilis and M. spirata (Labiatae) oils), (—)-isopulegol (E11) (in Mentha rotundifolia (Labiatae)), (—)-piperitol (E12) (in several Mentha and Eucalyptus species), (—)-α-terpineol (E13) (in Artemesia, Eucalyptus, Juniperus, and Mentha species), and (—)-carveol (E14). Oxidation products of both saturated and unsaturated cyclohexane monoterpene alcohols also occur in nature. Of these, the most abundant in the plant kingdom are (—)-menthone (E15) (in peppermint (Mentha x piperita) oil), (-)-isopulegone (E16) (in oil of Mentha pulegium), (—)-piperitone (E17) (in Eucalyptus oil), and (+)-carvone (E18) (in ripe fruits of dill (Anethum graveolens, Umbelliferae) and caraway (Carum carvi, Umbelliferae)).

Figure 5. Chemical diversity of monocyclic cyclohexane and aromatic monoterpenes.
Aromatic versions of cyclohexane monoterpenes (benzenoid menthanes or cymenes) are also found in nature and are constituents of some plants frequently used as spices. The hydrocarbon p-cymene (E19) has been found to occur in the oils of cinnamon (Cinnamonum zeylanicum), cypress, eucalyptus, thyme, and turpentine, whereas m-cymene (E20) is a constituent of the oil of blackcurrant (Ribes nigrum, Saxifragaceae). The corresponding phenols, thymol (p-cymen-3-ol) (E21) and carvacrol (p-cymen-2-ol) (E22), have been found to occur in many plants. Thymol (E21) is a constituent of thyme (Thymus vulgaris, Labiatae) and Orthodon angustifolium (Labiatae). Carvacrol (E22) has been found to occur in oils of thyme, marjoram, origanum, and summer savoy.
Additional chemical diversity of monoterpenes is apparent from the natural occurrence of their bicyclic analogs that bear cyclopropane (carane and thujane types), cyclobutane (pinane type), and cyclopentane (camphene/bornane, isocamphane and fenchone types) rings (Figs. 6 and 7). The carane type of bicyclic monoterpenoids in plants is represented by (+)-3-carene (F1) that occurs in Pinus longifolia (Pinaceae) and the related carboxylic acid, (+)-chaminic acid (F2), in Chamaecyparis nootkatensis (Cupressaceae). Compared with caranes, the thujane type of monoterpenoids is more abundant in plants. The hydrocarbon analog (—)-3-thujene (F3) has been found to occur in the oils of coriander (Coriandrum sativum), Eucalyptus , and Thuja occidentalis (Cupressaceae). Its regioisomer, (+)-sabinene (F4), occurs in Juniperus sabina (Cupressaceae). The hydration product of (—)-3-thujene, namely (—)-thujol (F5), occurs in plants belonging to the genera Thuja, Artemesia, and Juniperus, whereas the corresponding ketone (+)-3-thujanone (F6) is found in the oils of several plants of the families Asteraceae, Labiatae, and Pinaceae.

Figure 6. Bicyclic cyclopropane and cyclobutane monoterpenoids.

Figure 7. Bicyclic cyclopentane monoterpenoids.
Pinane-type bicyclic monoterpenoids (Fig. 6) occur in the wood of several species of Pinus. The most abundant are α- and β-pinenes (F7 and F8, respectively). Allylic hydroxylation products of pinenes, (+)-verbenol (F9), (+)-myrtenol (F10), and (—)-pinocarveol (F11) also occur in nature together with their products of oxidation: (+)-verbenone (F12), (+)-myrtenal (F13), and (—)-pinocarvone (F14). (+)-Verbenol is a constituent of the oil of turpentine. Its regioisomers, (+)-myrtenol and (—)-pinocarveol, occur in oils of orange (Citrus sinensis, Rutaceae) and eucalyptus (Eucalyptus globulus, Myrtaceae), respectively.
Cyclopentane bicyclic monoterpenoids that occur in the plant kingdom belong to three major skeletal types: camphane, isocamphane, and fenchane (Fig. 7). Camphane-type terpenoid alcohols, (+)-borneol (G1) and (—)-isoborneol (G2), have been isolated from Cinnamomum camphora (Lauraceae) and Achillea filipendulina (Asteraceae). A ketone derived from these, (+)-camphor (G3), is found in the camphor tree (Cinnamomum camphora) and in the leaves of rosemary (Rosmarinus officinalis) and sage (Salvia officinalis, Labiatae). Camphene (G4) and its enantiomer with the isocamphane carbon skeleton are known to occur in the oils of citronella and turpentine. Fenchane-type bicyclic cyclopentane monoterpenoids are commonly found in plants as their ketone derivatives. (—)-Fenchone (G5) occurs in the tree of life (Thuja occidentalis, Cupressaceae). Its enantiomer, (+)-fenchone (G6), has been isolated from the oil of fennel (Foeniculum vulgare, Umbelliferae).
Sesquiterpenoids
The C15 terpenoids known as sesquiterpenoids are the most chemically diverse group of terpenoids known in nature. Like monoterpenoids, many sesquiterpenoids contribute to the flavor and fragrances of a variety of plant products. To date about 10,000 sesquiterpenoids are known (32), and in the plant kingdom they commonly occur as hydrocarbons, alcohols, aldehydes, ketones, carboxylic acids, lactones, and oxiranes. The acyclic sesquiterpene hydrocarbons α- and β-farnesenes (H1 and H2, respectively) (Fig. 8) are constituents of the oils of orange (Citrus sinensis, Rutaceae) and mandarin (Citrus aurantium and C. reticulata, Rutaceae). The parent alcohol (E, E)-farnesol (H3) occurs in Acacia farnensiana (Mimosaceae), and its regiosiomer (S)-(+)-nerolidol (H4) is a constituent of the oil of neroli obtained from orange flowers. Some farnesane derivatives that contain furan rings also occur in nature, and these include dendrolasin (H5) from sweet potato (Ipomoea batatas, Convolvulaceae) and longifolin (H6) from the leaves of Actinodaphe longifolia (Lauraceae).

Figure 8. Acyclic plant sesquiterpenoids and their derivatives.
The vast chemical diversity of cyclic sesquiterpenoids compared with monoterpenoids results from the number of possible cyclization modes that is enhanced because of increased chain length and the presence of additional double bonds in the acyclic precursor farnesyl diphosphate (FPP). As depicted in Fig. 9, these cyclization modes lead to sesquiterpenoids with mono-, bi-, and tricyclic structures. The cyclofarnesane, (S)-(+)-abscisic acid (I1) (Fig. 10), an antagonist of plant growth hormones essential for plants in controlling flowering, shedding of leaves, and falling of fruits, is a monocyclic sesquiterpene formed because of bond formation between C-6 and C-11. Other common ring closures occur because of bond formation between C-1 and C-6, C-1 and C-10, and C-1 and C-11, which gives rise to bisabolane, germacrane, and humulane types of monocyclic sesquiterpenes (Fig. 9). Over 100 bisabolanes, 300 germacranes, and 30 humulanes are known to occur in nature (32). The elemane type of monocyclic sesquiterpenes is structurally related to germacranes as they can arise by a COPE rearrangement involving bond formation between C-1 and C-6 followed by the cleavage of the bond between C-8 and C-9. To date about 50 elemane-type monocyclic sesquiterpenes are known (32). Among the monocyclic sesquiterpenoids, bisabolanes represent one of the important classes known to occur in plants. Common examples of bisabolane in plants include (+)-β-bisabolene (I2), β-sesquiphellandrene (I3), and (—)-zingiberene (I4) in the rhizome of ginger (Zingiber officinalis, Zingiberaceae) and sesquisabinene (I5) from pepper (Piper nigrum, Piperaceae). Germacrane-type sesquiterpenoids contain a 10-membered macrocyclic ring; many of these sesquiterpenoids are constituents of essential oils derived from plants. The germacrane hydrocarbons, germacrenes B (I6) and D (I7), are found in Citrus junos and C. bergamia (Rutaceae), respectively. Some elemane-type sesquiterpenoids present in plants are represented by (—)-bicycloelemene (I8) from peppermint (Mentha piperita and M. arvensis) and β-elemenone (I9) from Commiphora abyssinica. The humulane type of monocyclic sesquiterpenes, which contain an 11-membered macrocyclic ring, is also found in plants and includes regioisomeric α- and β-humulenes (I10 and I11, respectively) from Lindera strychnifolia (Lauraceae), and (—)-humulol (I12), all of which are important constituents of the essential oils from cloves (Caryophylli flos, Caryophyllaceae), hops (Humulus lupulus, Cannabaceae), and ginger (Zingiber zerumbeticum, Zingiberaceae). Bicyclic sesquiterpenoids are formed because of two carbon-carbon bonds, each linking two carbon atoms of the farnesane skeleton together.
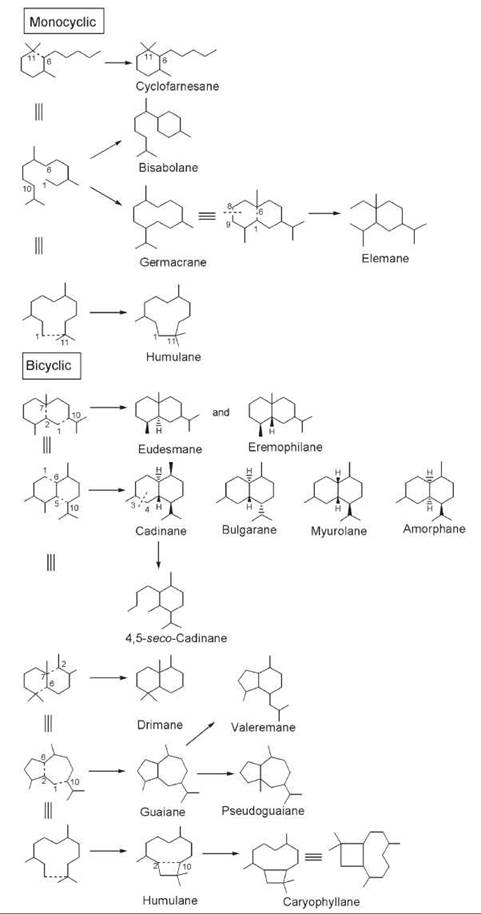
Figure 9. Cyclization modes of farnesane skeleton that lead to diverse monocyclic and bicyclic sesquiterpenoids.

Figure 10. Chemical diversity of monocyclic sesquiterpenoids.
Diterpenoids
The diterpenoids, which contain 20 carbon atoms, are represented by acyclic, monocyclic, bicyclic, tricyclic, and tetracyclic structures. Over 5,000 naturally occurring diterpenoids, many of which frequently occur in plant families Araliaceae, Asteraceae, Cistaceae, Cupressaceae, Euphorbiaceae, Leguminosae, Labiatae, and Pinaceae, are known (32). The acyclic diterpenoid alcohol phytol (J1) (Fig. 11) is a part of the structure of chlorophyll. A group of monocyclic diterpenoids with a 14-carbon macrocyclic ring called cembranes [e.g., cembrene A (J2)] also occurs in plants and is represented by over 100 members (32). Among the other cyclic diterpenoids, the most abundant in plants are the bicyclic labdanes [e.g., (—)-forskolin (J3)], tricyclic abietanes [e.g., abietic acid (J4)], and tetracyclic kauranes [e.g., (—)-kaurane (J5)], represented respectively by 500, 200, and 100 members (32). Baccatin III (J6), which is the diterpenoid part of the well-known anticancer drug paclitaxel (Taxol®; Mead Johnson, Princeton, NJ), contains a tricyclic skeleton derived from cembrane (see above).

Figure 11. Some representative examples of diterpenoids in plants.
Triterpenoids and steroids
Triterpenoids and steroids are groups of natural products that contain about 30 carbon atoms. They have a common origin, and their structures can be considered as being derived from that of squalene. Triterpenoids are found mostly in the plant kingdom, whereas steroids occur in plants, animals, and microorganisms. The chemical diversity of plant triterpenoids results from the ability of the C30 precursor, squalene, to undergo various modes of cyclization and subsequent “decoration” reactions. The plant triterpenoids belong to two main groups, the tetracyclic and pentacyclic. The tetracyclic triterpenoids, which consist of dammarane (K1) and tirucallane (K2) among others, are regarded by some authors as methylated steroids. The group of pentacyclic triterpenoids is by far the most diverse and is divided into five main groups: friedelane (K3), lupane (K4), ursane (K5), oleanane (K6), and hopane (K7) (Fig. 12). The steroids are modified triterpenoids that contain the tetracyclic ring system present in lanosterol. Chemical diversity represented by steroids depends mainly on the nature of the side chain attached to the steroid nucleus. Most prevalent in the plant kingdom are stigmastane (K8) and cycloartane (K9) classes of steroids (Fig. 12).
Triterpenoids and steroids frequently occur in many plant species as their glycosides called saponins (33). The chemical diversity of saponins is dependant therefore on both the nature of the 30-carbon moiety and the carbohydrate residue. Some saponins contain carbohydrate residues attached to several different positions of the aglycone (triterpenoid or the steroid) skeleton. Saponins are classified into 11 main structural classes based on the carbon skeletons of their aglycone moiety (33). In addition to their soap-like behavior in aqueous solution because of this combination of polar (carbohydrate) and nonpolar (aglycone) structural elements, saponins exhibit a diverse range of pharmacological and medicinal properties. Within the plant kingdom saponins are present in two major taxonomic classes, Magneliopsida (dicot) and Liliopsida (monocot) (33). Some important examples of saponins include glycyrrhizic acid (K10) from licorice and digitoxin (K11) from foxglove (Digitalis purpurea).

Figure 12. Triterpenoid and steroid skeletons common in plants and structures of some saponins.
Products of Shikimate Pathway
The shikimate pathway links the metabolism of carbohydrates to the biosynthesis of aromatic natural products via aromatic amino acids. This pathway, which is found only in plants and microorganisms, provides a major route to aromatic and phenolic natural products in plants. To date, over 8,000 phenolic natural products are known, which accounts for about 40% of organic carbon circulating in the biosphere. Although the bulk of plant phenolics are components of cell wall structures, many phenolic natural products are known to play functional roles that are essential for the survival of plants.
It has been noted that the chemical diversity of plant phenolics is as vast as the plant diversity itself. Most plant phenolics are derived directly from the shikimic acid (simple benzoic acids), shikimate (phenylpropanoid) pathway, or a combination of shikimate and acetate (phenylpropanoid-acetate) pathways. Products of each of these pathways undergo additional structural elaborations that result in a vast array of plant phenolics such as simple benzoic acid and cinnamic acid derivatives, monolignols, lignans and lignin, phenylpropenes, coumarins, stilbenes, flavonoids, anthocyanidins, and isoflavonoids.
Benzoic acid derivatives
As apparent from their structures, many benzoic acid derivatives are directly formed from shikimic acid by dehydration, dehydrogenation, and enolization reactions. Gallic acid is a component of gallotannins common in some plants that are used in the tanning of animal hides to make leather. Astringency of some foods and beverages, especially coffee, tea, and wines, is because of their constituent tannins. Other benzoic acid derivatives that occur in plants include protocatechuic acid, 4-hydroxybenzoic acid, and salicylic acid.
Cinnamic acid derivatives
Cinnamic acid and its derivatives found in plants originate from the aromatic amino acids L-phenylalanine and L-tyrosine by the elimination of ammonia. Some common natural cinnamic acid derivatives include p-coumaric acid, caffeic acid, ferulic acid, and sinapic acid.
Monolignols, lignans, and lignin
The alcohols formed from some cinnamic acid derivatives, namely p-coumaryl alcohol, coniferyl alcohol (L1), and sinapyl alcohol (L2), commonly known as monolignols, undergo dimerization reactions that yield lignans such as (+)-pinoresinol (L3), (+)-sesamin (L4), (—)-matairesinol (L5), and podophyllotoxin (L6) (Fig. 13). Several thousand lignans are found to occur in nature. Lignins, the structural components of plant cell walls, are polymers of monolignols and/or lignans.

Figure 13. Phenyl propanoids of plant origin.
Phenylpropenes
Phenylpropenes are derived from cinnamic acid and its derivatives by a series of reductions and other transformations. Cinnamaldehyde, the first product of the reduction of cinnamic acid, occurs in the bark of cinnamon (Cinnamomum zeylanicum, Lauraceae). Several hydrocarbon analogs are also known to occur in plants. Anethole is the main constituent of oils from aniseed (Pimpinella anisum, Umbelliferae), fennel (Foeniculum vulgare, Umbelliferae), and star anise (Illicium varum, Illiciaceae). Eugenol is a major constituent of cinnamon leaf, whereas myristicin occurs in nutmeg (Myristica fragrans, Myristicaceae).
Coumarins
Coumarins derive their name from their precursor, o-coumaric acid. They occur widely in plants both in the free form and as glycosides and are commonly found in families such as the Um- belliferae and Rutaceae. The parent compound coumarin (M1) is found in sweet clover (Melilotus alba, Leguminosae) and its hydroxyl derivative umbelliferone (M2) has been isolated from several Ferula spp. (Umbelliferae). Coumarins with complex structures also occur in plants and are formed by incorporating additional carbons derived from the mevalonate pathway. Alkylation of umbelliferone (M2) with dimethylallyl diphosphate (DMAPP) leads to demethylsuberosin (M3), which undergoes cyclization yielding marmesin (M4), the precursor of naturally occurring furanocoumarins, psorolen (M5), and bergapten (M6) (Fig. 14).

Figure 14. Coumarins, stilbenes, flavonoids, anthocyanidins, and isoflavonoids of plant origin.
Stilbenes, flavonoids, anthocyanidins, and isoflavonoids
In contrast to other plant phenolics, the basic carbon skeleton of stilbenes, flavonoids, anthocyanidins, and isoflavonoids incorporates elements of both shikimate (phenylpropanoid) and acetate pathways. Plant phenolics derived from this mixed biosynthetic pathway include the well-known cancer chemopre-ventative stilbene, resveratrol (M7), present in wine; naringenin (M8), a flavonone from Heliotropium and Nonea spp. (Boraginaceae); apigenin (M9), a flavone from German chamomile (Matricaria recuitita, Asteraceae); rutin (M10), a flavonol glycoside from several plants including hawthorn (Crataegus spp., Rosaceae); pelargonidin (M11), an anthocyanidin responsible for brilliant colors of many flowers; and genistein (M12) (Fig. 14), an isoflavonoid with oestrogenic activity and thus referred to as a phyto-oestrogen.
Alkaloids
Alkaloids constitute nitrogen-containing natural product bases that occur mainly in plants. About 20% of the flowering plant species are known to produce alkaloids (6). To date, over 12,000 plant-derived alkaloids have been reported, and they are grouped into various classes based on their origin and the nature of the nitrogen-containing moiety. Alkaloids commonly originate from the amino acids, L-ornithine, lysine, nicotinic acid, tyrosine, phenylalanine, tryptophan, anthranilic acid, and histidine, and thus contain pyrrolidine, pyrrolizidine, piperidine, quinolizidine, indolizidine, pyridine, quinoline, isoquinoline, indole, and imidazole ring systems. Alkaloids are also known to originate from mixed biosynthetic pathways, the most important of which include terpenoid and steroidal alkaloids. A limited number of alkaloids that contain a purine ring (e.g., caffeine) also occur in plants. Of the large number and variety of plant alkaloids, only a few are considered here for the purpose of illustration of their chemical diversity.
Alkaloids derived from aliphatic amino acids and nicotinic acid
Alkaloids that contain pyrrolidine and pyrrolizidine ring systems are derived from the nonprotein amino acid, L-ornithine. Cocaine (N1) and (—)-hyoscyamine, the two important pyrrolidine alkaloids that contain a tropane ring system, have been found to occur in coca (Erythroxylon coca, Erythroxylaceae) leaves and the whole plant of the deadly nightshade (Atropa belladonna, Solanaceae). The hepatotoxic alkaloid senecionine (N2) contains a bicyclic pyrrolizidine skeleton derived from two molecules of L-ornithine.
Piperidine alkaloids, for example, piperine (N3), and pseudopelletierine, are known to be derived from the amino acid, L-lysine. Piperine is responsible for the pungency of black pepper (Piper nigrum, Piperaceae), whereas pseudopelletierine is a constituent of the bark of pomegranate (Punica granatum, Punicaceae). The bicyclic ring system in quinolizidine alkaloids such as (—)-sparteine (N4) in the broom plant (Cytisus scoparius, Leguminosae) is derived from two molecules of L-lysine in a manner similar to the L-ornithine-derived pyrrolizidine ring system. Indolizidine alkaloids derived from L-lysine via the cyclic amino acid, L-pipecolic acid, contain fused six- and five-membered rings with a nitrogen atom at the ring fusion. An important example of an indolizidine alkaloid is swainsonine (N5), which occurs in the leguminous plant Swainsonia canescens. Alkaloids that contain a pyridine ring also occur in the plant kingdom. Two common plant-derived pyridine alkaloids, nicotine (N6) and anabasine, both of which are found in tobacco (Nicotiana tabacum, Solanaceae), contain a pyridine and a pyrrolidine or a piperidine ring, respectively (Fig. 15).

Figure 15. Chemical diversity of plant alkaloids.
Alkaloids derived from aromatic amino acids
Aromatic amino acids that originate from the shikimate pathway also act as precursors to many alkaloids. Alkaloids that contain a phenylethylamine moiety are derived from L-tyrosine or its oxidation product L-dihydroxyphenylalanine (L-DOPA). Mescaline (N7) originating from the latter amino acid is known to occur in several cacti and is responsible for the hallucinogenic activity of peyote (Lophophora williamsii, Cactaceae). Lophocerine is a tetrahydroisoquinoline alkaloid derived from L-dopamine and found to occur in a different Lophophora species, L. schotti.
Condensation of two phenylethyl units derived independently from the same or different aromatic amino acid(s) leads to a variety of benzyl-tetrahydroisoquinolines, which, with additional structural modifications, produce a diverse range of alkaloids. (S)-Reticuline occurring in several plant species of Annonaceae is an important benzyl-tetrahydroisoquinoline alkaloid that acts as a precursor to several pharmacologically active alkaloids such as papaverine (N8), (+)-tubocurarine (N9), and morphine (N10). Papaverine and morphine are known to occur in opium (Papaver somniferum, Papaveraceae) and are responsible for its narcotic activity, whereas (+)-tubocurarine (N9) is a muscle relaxant obtained from the arrow poison of the South American Indians, curare (Chondrodendron tomentosum, Menispermaceae). Phenethylisoquinoline alkaloids are similar structurally to benzylisoquinolines but as the name implies contain a phenylethyl moiety instead of a benzyl moiety as the pendant aromatic ring. Both (S)-autumnaline and the cyclized analog colchicine (N11) belonging to this class have been found to occur in the seeds of autumn crocus (Colchicium autumnale, Liliaceae).
Maximization of Chemical Diversity and Production of Natural Products in Plants
As is apparent from the foregoing discussion, plants produce a huge array of natural products, many of which are specialized secondary metabolites associated with particular plant species and/or having to play important ecological roles. It is likely that for diversification and survival of the plant kingdom, individual plants had to develop the ability to perform in vivo combinatorial chemistry by mixing and matching and evolving the genes required for different secondary metabolite biosynthetic pathways (34, 35). With the elucidation of several secondary metabolic pathways in plants together with the advent of techniques for the introduction of genes into plants and the availability of an increasing number of genes, it has become possible to modulate and diversify secondary metabolite production in transgenic plants and plant cell cultures.
Two general approaches for the production of long-chain polyunsaturated fatty acids usually found in fish oil have been employed, both of which used 18 carbon fatty acids endogenous to plants as the starting substrates (36). Soybean and canola, the oilseed plants rich in omega-6 fatty acids, have been engineered to produce omega-3 polyunsaturated fatty acids such as eicosapentaenoic acid (EPA) and docosohexaenoic acid (DHA) (37, 38).
Chalcone synthase (CHS), the first plant natural product polyketide synthase (PKS) to be characterized at the molecular level (39), catalyzes the condensation of 4-coumaroyl-CoA with three molecules of malonyl-CoA to afford naringenin chalcone, a precursor of the major classes of plant flavonoids. The cloning of a novel type III pentaketide chromone synthase (PCS) from aloe (Aloe arborescens, Liliaceae) rich in aromatic polyketides, especially quinones such as aloe-emodin and emodin, resulted in PCS-catalyzed condensation of five molecules of malonyl-CoA to produce 5,7-dihydroxy-2-methyl chromone new to this plant (40). Another novel Aloe arborescens type III PKS that produces two hitherto unknown aromatic octaketides, SEK4 and SEK4b, has recently been reported (41). The application of plant cell cultures for the production of the polyketide hypericin from St. John’s wort (Hypericum performatum, Hypericaceae) has been investigated (42).
To date over 30 plant terpenoid synthases have been cloned as cDNAs, and many of these were found to encode enzymes of secondary metabolism (43). Isolation and analysis of six genomic clones encoding monoterpene ((-)-pinene and (—)-limonene), sesquiterpene ((E)-α-bisabolene and S-selinene) and diterpene (abietadiene) synthases from Abies grandis, and a diterpene (taxadiene) synthase from Taxus brevifolia have been reported (44). Overexpression of a cotton farnesyl diphosphate synthase (FPPS) in transgenic Artemesia annua has resulted in 3- to 4-fold increase in the yield of the sesquiterpenoid antimalarial drug, artemisinin, in hairy roots (45).
Plant cell culture, an environmentally friendly and renewable alternative for the production of plant natural products, has also been investigated to obtain taxane diterpenoids from Taxus sp. (46-48) and terpene indole alkaloids from the Madagascar periwinkle (Catharanthus roseus) (49). A recent study has provided evidence for the production of novel terpene indole alkaloids using both differentiated Catharanthus roseus (seedlings) and hairy root culture (50).
References
1. Pimm SL, Russell GJ, Gittleman JL, Brooks TM. The future of biodiversity. Science 1995; 269:347-350.
2. Lewis NG, Davin LB. Evolution of lignan and neolignan biochemical pathways. In: Isoprenoids and other natural products: Evolution and function. Nes WD, ed. 1994. ACS Symposium Series, Washington, D.C.
3. Kinghorn AD. Pharmacognosy in the 21st Century. J. Pharm. Pharmacol. 2001; 53:135-148.
4. Dixon RA. Natural products and plant disease resistance. Nature 2001; 411:843-847.
5. Verpoorte R. Exploration of nature’s chemodiversity: the role of secondary metabolites as leads in drug development. Drug Discov. Today 1998; 3:232-238.
6. Croteau R, Kutchan TM, Lewis NG. Natural Products (Secondary Metabolites). In: Biochemistry and Molecular Biology of Plants, Fifth Impression. Buchanan BB, Gruissem W, Jones RL, eds. 2005. American Society for Plant Physiologists, Rockville, Maryland.
7. Pietra F. Biodiversity and natural product diversity. 1st edition. 2002. Elsevier Science, Netherlands.
8. Romeo JT, Saunders JA, Barbosa P, eds. Recent Advances in Phytochemistry, Vol 30. Phytochemical Diversity and Redundancy in Ecological Interactions. 1996. Plenum Press, New York.
9. Hartmann, T. Plant-derived secondary metabolites as defensive chemicals in herbivorous insects: a case study in chemical ecology. Planta 2004; 219:1-4.
10. Firn RD, Jones CG. Natural products - a simple model to explain chemical diversity. Nat. Prod. Rep. 2003; 20:382-391.
11. Tulp M, Bohlin L. Functional versus chemical diversity: is biodiversity important for drug discovery? Trends Pharmacol. Sci. 2002; 23:225-231.
12. Tulp M, Bohlin L. Chemical diversity: independent of functional diversity. Trends Pharmacol. Sci. 2002; 23:405.
13. McKey D. The distribution of secondary compounds within plants. In: Herbivores: Their Interaction with Plant Secondary Metabolites. Rosenthal G, Janzen D, eds. 1979. Academic Press, New York.
14. Feeny P. The evolution of chemical ecology: contributions from the study of herbivorous insects. In: Herbivores: Their Interactions with Secondary Plant Metabolites. Rosenthal A, Berenbaum MR, eds. 1992. Academic Press, San Diego.
15. Futuyama DJ, Keese Mc. Evolution and coevolution of plants and phytophagous arthropods. In: Herbivores: Their Interactions with Secondary Plant Metabolites Rosenthal A, Berenbaum MR, eds. 1992. Academic Press, San Diego.
16. Harborne JB. Twenty-five years of chemical ecology. Nat. Prod. Rep. 2001; 18:361-379.
17. Jones CG, Firn RD. On the evolution of secondary plant chemical diversity. Philos. Trans. R. Soc. 1991; 333:273-280.
18. Firn RD, Jones CG. Do we need a new hypothesis to explain plant VOC emissions? Trends Plant Sci. 2006; 11:112-113.
19. Weissman KJ, Leadlay PF. Combinatorial biosynthesis of reduced polyketides. Nat. Rev. Microbiol. 2005; 3:925-936.
20. Aitzetmuller K, Matthaus B, Friedrich H. A new database for seed oil fatty acids - the database SOFA. Eur. J. Lipid Sci. Technol. 2003; 105:92-103.
21. Viegas C Jr, de Rezende A, Silva DHS, Castro-Gamboa I, Bolzani V da S, Barreiro EJ, Palhares de Miranda AL, Alexandre-Moreira MS, Young MCM. Ethnopharmacological, biological and chemical aspects of the Cassia genus. Quim. Nova 2006; 29:1279-1286.
22. Fairbairn JW. The analysis and standardization of anthraquinone drugs. Planta Med. 1964; 12:260-264.
23. Reynolds T. Aloe chemistry. Curr. Topics Phytochem. 2002; 5:235-254.
24. Miskovsky P. Hypericin - a new antiviral and antitumor photosensitizer: mechanism of action and interaction with biological macromolecules. Curr. Drug Targets 2002; 3:55-84.
25. Rohdich F, Bacher A, Eisenreich W. Isoprenoid biosynthetic pathways as anti-infective drug targets. Biochem. Soc. Trans. 2005; 33:785-791.
26. Withers ST, Keasling JD. Biosynthesis and engineering of iso- prenoids small molecules. Appl. Microbiol. Biotechnol. 2007; 73:980-990.
27. Lee S. Artemisinin, promising lead natural product for various drug developments. Mini Rev. Med. Chem. 2007; 7:411-422.
28. Kingston DGI. Taxol and Its Analogs. In: Anticancer Agents from Natural Products. Cragg GM, Kingston DGI, Newman DJ, eds. 2005. Taylor & Francis, Boca Raton, FL.
29. Wilms K. Chemistry and mechanism of Vinca alkaloids. Planta Med. 1972; 22:324-333.
30. Gueritte F, Fahy J. The Vinca Alkaloids. In: Anticancer Agents from Natural Products. Cragg GM, Kingston DGI, Newman DJ, eds. 2005. Taylor & Francis, Boca Raton, FL.
31. Dembitsky VM. Astonishing diversity of natural surfactants: 7. Biologically active hemi- and monoterpenoid glycosides. Lipids 2006; 41:1-27.
32. Connolly JD, Hill RA. Dictionary of Terpenoids, Vols. 1-3. 1991. Chapman and Hall, London.
33. Vincken JP, Heng L, de Groot A, Gruppen H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007; 68:275-297.
34. Osboure AE. Metabolic diversity in plants. In Proc. BCPC International Congress: Crop Science and Technology. Brit. Crop Protection Council. 2005. pp. 979-984.
35. Bakht S, Field B, Inagaki Y, Jenner H, Melton R, Mylona P, Qi X, Qin B, Townsend B, Wegel E, Osbourne A. Metabolic diversity in plants. Biol. Plant Microbe Interact. 2006; 5:107-112.
36. Graham IA, Larson T, Napier JA. Rational metabolic engineering of transgenic plants for biosynthesis of omega-3 polyunsaturates. Curr. Opin. Biotechnol. 2007; 18:142-147.
37. Damude HG, Kinney AJ. Engineering oilseed plants for a sustainable, land-based source of long chain polyunsaturated fatty acids. Lipids 2007; 42:179-185.
38. Napier JA. The production of unusual fatty acids in transgenic plants. Annu. Rev. Plant Biol. 2007; 58:295-319.
39. Kreuzaler F, Ragg H, Fautz E, Kuhn DN, Hahlbrook K. UV- induction of chalcone synthase mRNA in cell suspension cultures of Petroselinum hortense. Proc. Natl. Acad. Sci. USA. 1983; 80:2591-2593.
40. Abe I, Utsumi Y, Oguro S, Morita H, Sano Y, Noguchi H. A plant type III polyketide sythase that produces pentaketide chromone. J. Am. Chem. Soc. 2005; 127:1362-1363.
41. Abe I, Oguro S, Utsumi Y, Sano Y, Noguchi H. Engineered biosynthesis of plant polyketides: chain length control in octaketide-producing plant type III polyketide synthase. J. Am. Chem. Soc. 2005; 127:12709-12716.
42. Kirakosyan A, Sirvent TM, Gibson DM, Kaufman PB. The production of hypericins and hyperforin by in vitro cultures of St. John’s wort (Hypericum perforatum). Biotechnol. Appl. Biochem. 2004; 39:71-81.
43. Bohlmann J, Meyer-Gaven G, Croteau R. Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc. Natl. Acad. Sci. USA. 1998; 95:4126-4133.
44. Trapp SC, Croteau RB. Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics 2001; 158:811-832.
45. Liu Y, Wang H, Ye HC, Li GF. Advances in the plant isoprenoid biosynthetic pathway and its metabolic engineering. J. Integr. Plant Biol. 2005; 47:769-782.
46. Frense D. Taxanes: perspectives for biotechnological production. Appl. Microbiol. Biotechnol. 2007; 73:1233-1240.
47. Tabata H. Paclitaxel production by plant cell culture technology. Adv. Biochem. Eng. Biotechnol. 2004; 87:1-23.
48. Tabata H. Production of paclitaxel and related taxanes by cell suspension cultures of Taxus species. Curr. Drug Targets 2006; 7:453-461.
49. Pasquali G, Porto DD, Fett-Neto AG. Metabolic engineering of cell cultures versus whole plant complexity in production of bioactive monoterpene indole alkaloids: recent progress related to an old dilemma. J. Biosci. Bioeng. 2006; 101:287-296.
50. McCoy E, O’Conner SE. Directed biosynthesis of alkaloid analogs in the medicinal plant Catharanthus roseus. J. Am. Chem. Soc. 2006; 128:14276-14277.
Further Reading
Breitmaier E. Terpenes flavors, fragrances, pharmaca, pheromones. 2006. Wiley-VCH Verlag GmbH & Co. KgaA, Weinheim. pp. 10-116.
Dewick PM. Medicinal natural products. A biosynthetic approach. 1997. John Wiley & Sons Inc., New York. pp. 32-440.
Hartmann T, Dierich B. Chemical diversity and variation of pyrrolizidine alkaloids of the senecionine type: biological need or coincidence? Planta 1998; 206:443-451.
Roberts SC. Production and engineering of terpenoids in plant cell culture. Nat. Chem. Biol. 2007; 3:387-395.
See Also
Natural Product Discovery, Molecular Biological Approaches to
Natural Products in Microbes, Chemical Diversity of
Natural Products: An Overview
Terpenoids in Plants