Chemistry for Dummies
Part IV. Chemistry in Everyday Life: Benefits and Problems
Chapter 16. Polymers: Making Big Ones from Little Ones
In This Chapter
· Understanding polymerization
· Differentiating the different types of plastics
· Finding out about recycling plastics
Ionce heard someone say that man never really invents anything new; he just copies nature. I’m not sure I believe that, with all the new inventions that have been developed recently. But I certainly think it’s true in the case of polymers. Nature has been building polymers forever. Proteins, cotton, wool, and cellulose are all polymers. They all fall into a class of compounds called macromolecules — very large molecules. Man has learned to produce macromolecules in the lab, changing the face of our society forever.
When I was a child, my father, very much a traditionalist, said that he wanted things made of metal, not that cheap imported plastic stuff. Wow, would he be shocked today. I’m surrounded by synthetic textiles (clothing and carpet, for example), I ride around in autos that are fast becoming cocoons of plastic, my home is filled with plastic bottles of all shapes, sizes, and hardness, and I have friends with knees or other parts that have been either replaced or enhanced with polymers. I cook with a skillet that has a nonstick surface, I use a nylon spatula, I watch a TV with a plastic case, and I go to sleep on a foam pillow. Our world is truly part of the Age of Plastics.
In this chapter, I show you how the process of polymerization takes place and how chemists go about designing polymers with certain desired characteristics. I also show you some different kinds of polymers and how they’re created. And I discuss some ways for getting rid of plastics before we bury ourselves in a mountain of milk jugs and disposable diapers. Welcome to the wonderful world of polymers!
Natural Monomers and Polymers
Nature has been building polymers for a long time. Cellulose (wood) and starch are prime examples of naturally occurring polymers. Take a look at Figure 16-1, which shows, the structures of cellulose and starch.
Notice anything similar about the two structures in the figure? They’re both made up of repeating units. In fact, the repeating unit in both cases is a glucose unit. Both starch and cellulose are natural macromolecules (large molecules), but they’re also examples of naturally occurring polymers, macromolecules in which there’s a repeating unit called a monomer. (Polymer should stand for “many mer.” The mer in this case is the monomer.) In the case of starch and cellulose, the monomer is a glucose unit. The structure of polymers is similar to taking a bunch of paper clips (monomers) and hooking them together to make a big long chain (polymer).
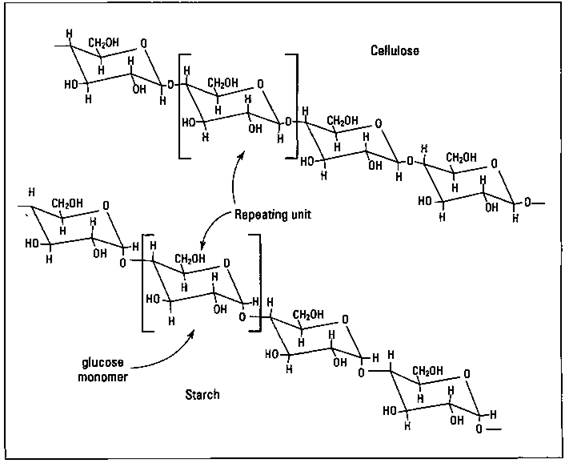
Figure 16-1: Cellulose and starch.
Notice another thing about cellulose and starch. The only way they differ is in how the glucose units are attached to each other. This minor change makes the difference between a potato and a tree. (Okay, it’s not quite that simple.) Human beings can digest (metabolize) starches but not cellulose. A termite can digest cellulose just fine. In natural polymers, just like in synthetic ones, a minor change sometimes makes a big difference in the properties of the polymer.
Classifying Unnatural (Synthetic) Monomers anti Polymers
Chemists took this idea of hooking together small units into very large ones from nature and developed a number of different ways of doing it in the lab. Now there are many different types of synthetic polymers. In this section, I introduce you to some of them and talk about their structures, properties, and uses.
Because chemists are big on grouping things together, they’ve put polymers into different classes. That works out just fine. Grouping gives chemists something to do and makes it easier for normal folks to get familiar with the various kinds of polymers out there.
We all need a little structure
One way of classifying polymers is by the structure of their polymer chain. Some polymers are linear. They’re composed of many long strands thrown together like pieces of rope. Branched polymers have short branches coming off the main polymer strand. Imagine taking those long pieces of rope and tying short pieces of rope to them along the entire length. Crosslinked polymers have the individual polymer chains linked together by side chains. Imagine taking those pieces of rope and making them into a hammock.
Feet the heat
Another way of classifying polymers is by their behavior under heat. Thermoplastic polymers become soft when they’re heated. Polymers of this type are composed of long linear or branched strands of monomer units hooked together. Have you ever left a pair of plastic sunglasses or a child’s plastic toy on the dashboard of your car in the middle of the summer? These plastics become really soft. Because they soften and melt, they can be remolded time and time again. This makes thermoplastics much easier to recycle. A vast majority of the plastics produced in the United States are of the thermoplastic type.
Thermosetting polymers don’t soften when heated, and they can’t be remolded. During production of this type of polymer, crosslinking (bridges between the polymer strands) is created in the plastic by heating it. Bakelite is a good example of a thermosetting plastic. It’s a hard, strong nonconductor. These properties make it ideal as an insulator and as a handle for frying pans and toasters.
Used and abused
A third way of classifying polymers is by their use by the consumer.
A plastic refers to the polymer’s ability to be molded. Whether they’re of the thermoplastic or thermosetting type, these polymers are molded during the manufacture of the end product. And they’re used to make our dishes, toys, and so on.
Fibers are linear strands held together by intermolecular forces such as hydrogen bonding between the polymer strands. These polymers are generally called textiles. They’re used to make our clothes and our carpets.
Elastomers, sometimes called rubber, are thermoplastic materials that become slightly crosslinked during their formation. Because of this, they stretch and bounce. Natural rubber (latex) is classified as an elastomer along with its synthetic counterparts. These types of polymers are used for things like latex gloves and rubber bands and balls.
Chemical process
One of the best ways of classifying synthetic polymers is by the chemical processes used to create them. These processes normally fall into one of two categories:
ü Addition polymerization
ü Condensation polymerization
Let’s hook up: Addition polymerization
Many of the common polymers you come into contact with every day are called addition polymers — polymers that are formed in a reaction called addition polymerization. In this type of reaction, all the atoms that start out in the monomer are incorporated into the polymer chain. The monomers involved in this type of polymerization normally have a carbon-to-carbon double bond that’s partially broken during polymerization. This broken bond forms a radical reactive site, or radicalwhich is a highly reactive atom that has an unpaired electron. The radical then gains an electron by joining up with another radical, and a chain is started, which eventually becomes the polymer. Scratching your head a bit? Looking at examples often helps folks understand chemical processes, so following are some examples of addition polymerization.
Polyethylene: Sandwich wrap and milk jugs
Polyethylene is the simplest of the addition polymers. It’s also one of the most economically important. Ethane is heated at a high temperature in the presence of a metal catalyst, like palladium. Ethane loses two atoms of hydrogen (which make hydrogen gas) and forms a double bond:
![]()
The ethylene (ethene) that’s produced here is the monomer used in the production of polyethylene. The ethylene is then subjected to high heat with a catalyst in the absence of air. The high heat and catalytic action causes one of the carbon-to-carbon double bonds (C=C) to break, with one electron going to each carbon. Both carbons now have an unpaired electron, so they become radicals. Radicals are extremely reactive and attempt to gain an electron. In terms of this polymerization reaction, the radicals can gain an electron by joining up with another radical to form a covalent bond. This happens at both ends of the molecule, and the chain begins to grow. Polyethylene molecules up to a molecular weight of 1 million grams/mol may be produced in this way (see Figure 16-2).
Different catalysts and pressures are used to control the structure of the final product. The polymerization of ethylene can yield three different types of polyethylene:
ü Low-density polyethylene (LDPE)
ü High-density polyethylene (HDPE)
ü Crosslinked polyethylene (CLPE)
Low-density polyethylene (LDPE) has some branches on the carbon chain, so it doesn’t pack together as closely and tightly as the linear polymer. It forms a tangled network of branched polymer strands. This type of polyethylene is soft and flexible. It can be used for food wrap, sandwich bags, grocery bags, and trash bags. And it, like all the forms of polyethylene, is resistant to chemicals.
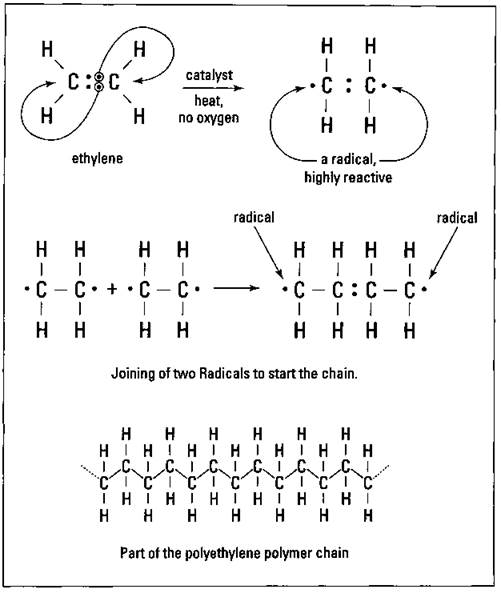
Figure 16-2: The addition polymerization of ethylene.
High-density polyethylene (HDPE) is composed of linear chains that are closely packed. This type of polymer is rigid, hard, and tough. Milk jugs, toys, and TV cabinets are made from HDPE. The Hula-Hoop was one of the first products ever made from this form of polyethylene.
Crosslinked polyethylene (CLPE) has crosslinking between the linear strands of monomers that are bonded together, producing a polymer that’s extremely tough. The lid on that HDPE milk jug is probably CLPE. Soft drink bottle caps are also CLPE. The soft drink bottles are made of another type of polymer that I discuss a little later in the chapter.
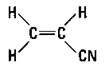
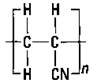
Polypropylene: Plastic ropes
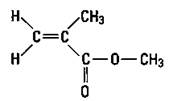
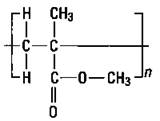
If you substitute another atom for a hydrogen atom on ethylene, you can produce a different polymer with different properties. If you substitute a methyl group for a hydrogen atom, you get propylene. Propylene, just like ethylene, has a double bond, so it can undergo addition polymerization in the same way as ethylene. The result is polypropylene (see Figure 16-3).
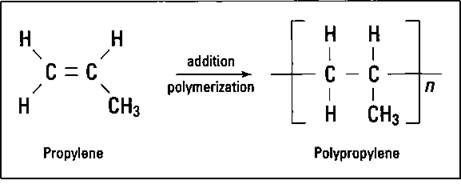
Figure 16-3: Propylene and polypropylene.
The small n in Figure 16-3 indicates that there are a number of the repeating units. Notice that this polymer has a methyl group side chain. Any time that the structure of a molecule is changed, the properties of the molecule change. By carefully adjusting the reaction conditions, chemists can construct polymers that have the side chains on the same side of the molecule, on alternating sides of the molecule, or distributed randomly. The position of these side chains changes the properties of the polymer somewhat so that polypropylene can be used for a wide variety of purposes, such as indoor- outdoor carpeting, battery cases, ropes, bottles, and automotive trim.
Polystyrene: Styrofoam cups
If you substitute a benzene ring for one of the hydrogen atoms on ethylene, you make styrene. Addition polymerization gives you polystyrene, as shown in Figure 16-4.
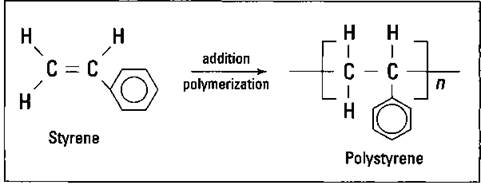
Figure 16-4: Styrene and polystyrene.
Polystyrene (Styrofoam) is a rigid polymer used for making foam drink cups, egg cartons, clear rigid drinking glasses, insulating materials, and packing materials. Environmentalists have criticized its use because it’s more difficult to recycle than some other plastics and is so widely used.
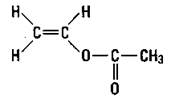
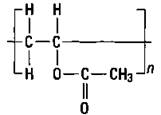
Polyvinyl chloride: Pipes and simulated leather
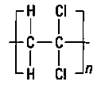
Substituting a chloride for one of the hydrogen atoms on ethylene gives you the vinyl chloride monomer that can polymerize to polyvinyl chloride (PVC), as shown in Figure 16-5.
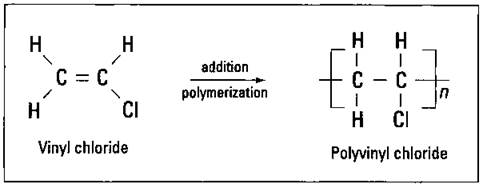
Figure 16-5: Vinyl chloride and polyvinyl chloride.
PVC is a tough polymer. It’s used extensively in rigid pipes of all types, flooring, garden hoses, and toys. Thin sheets of PVC used as simulated leather crack easily, so a plasticizer is added (a liquid that’s mixed with plastics to soften them and allow them to more closely resemble leather). However, after many years, plasticizers can evaporate from plastic, making it brittle and allowing it to crack.
Polytetrafluoroethylene: Stick stuff
Replace all the hydrogen atoms on ethylene with fluorine atoms, and you have tetrafluoroethylene. The tetrafluoroethylene can be polymerized to polytetrafluoroethylene, as shown in Figure 16-6.
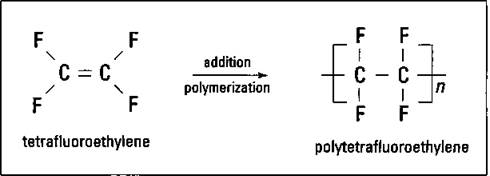
Figure 16-6: Tetrafluoroethylene and polytetrafluoroethylene.
Polytetrafluoroethylene is a material that’s hard, heat resistant, and extremely slick. This material is used as bearings, valve seats, and (most importantly to me) nonstick coating for pots and pans.
You can find some other addition polymers in Table 16-1.
Table 16-1. Other Addition Polymers
|
Monomer |
Polymer |
Uses |
|
Acrylonitrile |
Polyacrylonitrile |
Wigs, rugs, yarn |
|
Vinyl acetate |
Polyvinyl acetate |
Adhesives, latex, paint, chewing gum resin, textile coatings |
|
Methyl methacrylate |
Polymethyl methacrylate |
Contact lenses, glass substitute, bowling balls |
|
Vindylidine chloride |
Polyvindylidine chloride |
food wrap |
Let's get rid of something: Condensation polymerization
A reaction in which two chemical species combine with each other by eliminating a small molecule is called condensation polymerization. Polymers formed in this fashion are known as condensation polymers. Unlike in addition polymerization, no double bond is needed in this type of reaction.
A small molecule — normally water — is eliminated. Commonly, one molecule is an organic acid, and the other is an alcohol. These two molecules react, splitting off water and forming an organic compound called an ester. If a polymer chain grows, it forms a polyester.
Following are some examples of condensation polymers. These examples involve techno-speak specific to functional groups in organic chemistry, and they involve a lot of complexly named organic compounds. If you aren’t familiar with functional groups or how organic compounds are named, just flip to Chapter 14 for the details.
Polyester: Leisure suits and soft drink battles
If you take ethylene glycol, with its alcohol functional groups on both carbons, and react it with terephthalic acid, with its two organic acid functional groups, you can eliminate water and form the condensation polymer polyethylene terephthalate (PET), a polyester. Figure 16-7 shows the synthesis of PET.
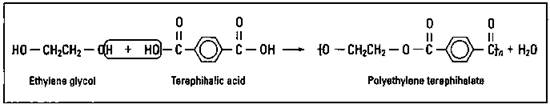
Figure 16-7: Synthesis of PET.
This is the polyester you find in clothing (boy, did I ever love that baby-blue leisure suit I had in the 70s!), artificial automotive tire cord, substitute blood vessels, film, and soft drink bottles.
Polyamides: Sheer enough for a woman, strong enough for a (police)man
If you react an organic acid with an amine, you split off water and form an amide. If you use an organic acid that contains two acid ends and an amine that has two amine ends (a diamine), then you can polymerize a polyamide. The polyamide is commonly referred to as nylon. Figure 16-8 shows the reaction between 1,6-hexanediamine and adipic acid to form Nylon 66. (The 66 indicates that there are 6 carbon atoms in both the amine and the organic acid.)
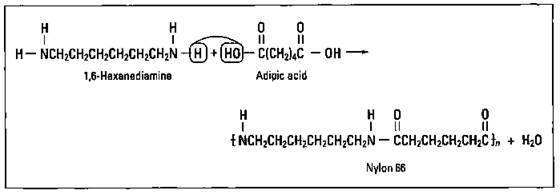
Figure 16-8: Synthesis of Nylon 66.
The synthesis of nylon in 1935 had a major impact on the textile industry. Nylon stockings first went on sale in 1939, and nylon was used in parachutes extensively during World War II. Make a slight substitution in one of the carbon backbones, and you have a material strong enough for a bullet-proof vest.
Silicones: Bigger and better
Because silicon is in the same family as carbon, chemists can produce a class of polymers that contains silicon in its structure. These polymers are known as silicones. Figure 16-9 shows the synthesis of a typical silicone.
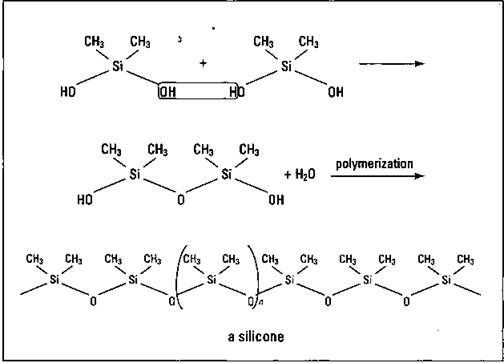
Figure 16-9: Synthesis of a silicone.
The silicone polymers are held together by the strong silicon-oxygen bond, and they can have molecular weights in the millions. They’re used as gaskets and seals, and they’re found in waxes, polishes, and surgical implants. The press has given the most attention to their use as surgical implants.
Silicone-based implants and prostheses have been used for years. They’ve been used as shunts, ear prostheses, finger joints, and, of course, breast implants. The implants themselves are filled with silicone oil. Occasionally, an implant leaks, and the silicone oil escapes into the body. In 1992, some evidence was found that silicone oil may trigger an autoimmune response. Although studies have not established a cause-and-effect relationship, many implants have been removed, and silicone oil is no longer used in the United States.
Polymers have reshaped our society as well as our figures. They’re useful in a very wide variety of ways, relatively inexpensive, and durable. Because they’re so durable, figuring out how to dispose of them is a major problem.
Reduce, Reuse, Recycle — Plastics
Plastics have basically an infinite lifetime. Nothing in nature does a good job at degrading them. If you bury that plastic plate, Styrofoam cup, or disposable diaper in a landfill and then dig it up ten years later, there will be no change. You could even dig it up a hundred years later and get the same results. Waste containing plastics will be with us for a long time.
Some plastics can be burned as fuels. They have a high heat content, but they often produce gases that are toxic or corrosive. Society can reduce its reliance on plastics to a certain degree. Using cardboard hamburger boxes and cellulose shipping packing instead of Styrofoam helps, but our best answer so far has been in the area of recycling.
Thermoplastic polymers can be melted down and reformed. But in order to do this, the plastics must be separated into their various components. Most plastic containers contain a symbol on the bottom that indicates what type of plastic the containers are made of. Recyclers can use these symbols to separate the plastics into various categories to make recycling easier. Figure 16-10 shows the recycling symbols for plastics and indicates what type of plastic each symbol represents.
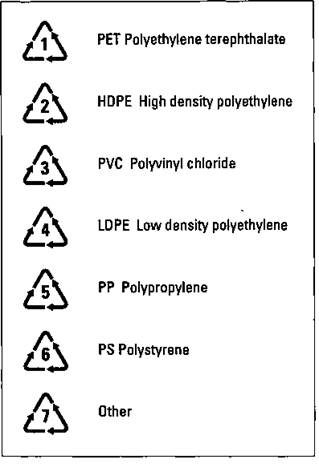
Figure 16-10: Plastic recycling codes.
PET bottles and HDPE milk jugs are probably the plastics recycled the most. But the major problem isn’t the chemistry involved in the recycling process: The major problems are encouraging individuals, families, and businesses to recycle and developing an easy means to collect and sort the plastics for recycling. These polymers are too valuable a resource to simply be buried in some landfill.