Chemistry for Dummies
Part IV. Chemistry in Everyday Life: Benefits and Problems
Chapter 19. Brown, Chunky Water? Water Pollution
In This Chapter
· Understanding where our water supply comes from
· Clarifying how the structure of water makes it vulnerable to pollution
· Taking a look at several types of water pollutants
· Finding out about water treatment
Water is absolutely necessary to our survival. After all, the human WW body is about 70 percent water. Most of the water on earth, however, is found as seawater. Only about 2 percent of the water on the earth is fresh water, and a little over three-quarters of that is in the form of ice and glaciers. But it’s that very small amount of fresh water suitable for drinking (potable water) that most people are concerned about.
I’m sure you’re quite aware of the water you drink and the water you use for bathing, cooking, and watering your lawn. But unless you live in a rural agricultural area, I doubt that you think much about the water used to grow the plants and animals we depend on for food.
In addition, water is used to carry waste products from our homes and to generate electricity. It’s also used in chemical reactions and cooling towers. And then there’s recreation — boating, swimming, and fishing. All these things depend on an adequate supply of good, pure water.
But where does water come from? How does it get contaminated, and how does it get cleaned? These are some of the questions I discuss in this chapter. So sit back, grab your glass of water, and dive in.
Where Does Our Water Come From, and Where Is It Going?
The actual amount of water on earth is relatively constant, but its location and purity may vary. Water moves throughout the environment by what is called the water cycle, or the hydrologic cycle. Figure 19-1 diagrams this cycle.
Evaporate, condense, repeat
Water evaporates (goes from a liquid to a gas when heated) from lakes, streams, oceans, trees, and even humans. As water evaporates, it leaves behind any contaminates that it may have accumulated. (That’s where the salt comes from on your sweatband and cap.) This process of evaporation is one of nature’s ways of purifying water.
The water vapor may then travel many miles, or it may stay relatively local, depending on the prevailing winds. Sooner or later, the vapor condenses (goes from a gas to a liquid when cooled) and falls back to the earth as rain, snow, or sleet.
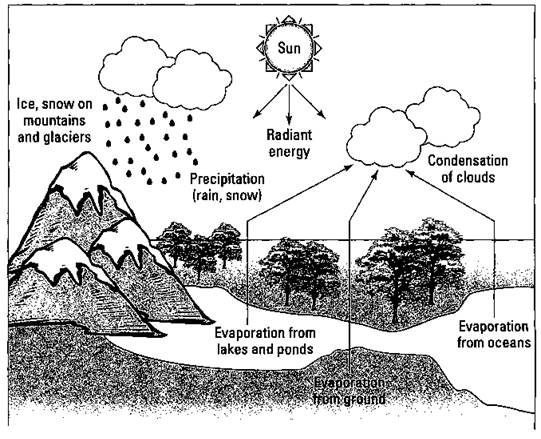
Figure 19-1: The water (hydrologic) cycle.
Where the water goes
Water may fall to earth and collect in a lake or stream. If it does, it eventually finds its way back to the sea. If it falls onto the land, it can form runoff end eventually enter a lake or stream, or it can soak into the ground and become groundwater. The porous layer of soil and rock that holds the groundwater forms a zone called an aquifer. This zone provides us with a good source of groundwater. We tap into these aquifers by using wells.
Human activities can affect this water cycle. Cutting vegetation can increase the rate of runoff, causing less water to become absorbed into the soil. Man-made dams and reservoirs increase the surface area available for water evaporation. Using more groundwater than can be replenished may deplete the aquifers and lead to water shortages. And society can contaminate the water in a wide variety of ways that I discuss in this chapter.
Water: A Most Unusual Substance
Water is a polar molecule. Chapter 7 covers polar molecules in detail, but here’s a quickie version relating to water: The oxygen in water (H2O) has a higher electronegativity (attraction for a bonding pair of electrons) them the hydrogen atoms, so the bonding electrons are pulled in closer to the oxygen. The oxygen end of the water molecule then acquires a partial negative charge, and the hydrogen atoms take on a partial positive charge. When the partially positively charged hydrogen (where’s my editor on that clunker of a phrase?) of one water molecule is attracted to the partially negatively charged oxygen of another water molecule, there can be a rather strong interaction between the water molecules. This interaction is called a hydrogen bond (H-bond). This is not to be confused with a hydrogen bomb. Two very different things. Figure 19-2 shows the hydrogen bonds that occur in water.
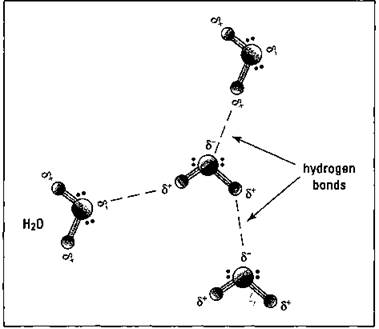
Figure 19-2: Hydrogen bonding in water.
Hydrogen bonds, caused by the polar covalent bonds of water molecules, give water some very unusual properties:
ü Water has a very high surface tension. The water molecules at the surface of the water are only attracted downward into the body of the liquid. The molecules in the body of the liquid, on the other hand, are attracted into all different directions. Bugs and small lizards can walk across water because they don’t exert enough force to break the surface tension. The high surface tension of water also means that evaporation rates are really less than you’d expect.
ü Water becomes a liquid at temperatures commonly found on earth. The boiling point of a liquid is normally related to its molecular weight. Substances that have molecular weights close to the molecular weight of water (18 g/mol) boil at far lower temperatures; these substances become gases at normal room temperature.
ü Ice, the solid state of water, floats when placed in water. Normally, you may think of a solid as having a higher density than its corresponding liquid, because the particles are closer together in the solid. When water freezes, however, it’s locked into a crystal lattice that has large holes incorporated into it by its hydrogen bonds. So the density of ice is less than that of water (see Figure 19-3). The floating property of ice is one of the reasons that life, in all its diversity and magnitude, is able to exist on earth. If ice were denser than water in the winter, the water at the top of lakes would freeze and sink. Then more water would freeze and sink, and so on. Pretty soon, the lake would be frozen solid, destroying most of the life — such as plants and fish — in the lake. Instead, ice floats and forms an insulating layer over the water, which allows life to exist, even in the winter.
ü Water has a relatively high heat capacity. The heat capacity of a substance is the amount of heat a substance can absorb or release in order to change its temperature 1 degree Celsius. Water’s heat capacity is almost 10 times greater than iron and 5 times greater than aluminum. This means that lakes and oceans can absorb and release large amounts of heat without a dramatic change in temperature, which moderates the temperature on earth. Lakes absorb heat during the day and release it at night. Without water’s high heat capacity, the earth would undergo dramatic swings in temperature during its day/night cycle.
ü Water has a high heat of vaporization. The heat of vaporization of a liquid is the amount of energy needed to convert a gram of the liquid to a gas. Water has a heat of vaporization of 54 calories per gram (see Chapter 2 for more about the calorie, a metric unit of heat). This high heat of vaporization allows us to rid our bodies of a great deal of heat when sweat is evaporated from the skin. This property also helps to keep the climate on earth relatively moderate without extreme short-term swings.
ü Water is an excellent solvent for a large number of substances. In fact, water is sometimes called the universal solvent, because it dissolves so many things. Water is a polar molecule, so it acts as a solvent for polar solutes. It dissolves ionic substances easily; the negative ends of the water molecules surround the cations (positively charged ions), while the positive ends of the water molecules surround the anions (negatively charged ions). (Turn to Chapter 6 for specifics on ions, cations, and anions.) With the same process, water can dissolve many polar covalent compounds, such as alcohols and sugars (see Chapter 7 for more on these types of compounds). This is a desirable property, but it also means that water dissolves many substances that are not desirable to us or that make water unusable. We lump all those substances together under the terms pollutants or contaminants.
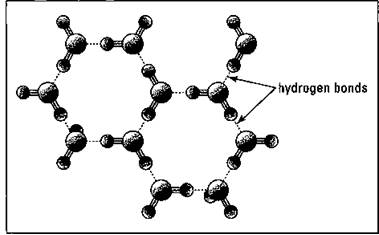
Figure 19-3: The structure of ice.
Yuck! Some Common Water Pollutants
Because water is such an excellent solvent, it easily picks up unwanted substances from a variety of sources. Figure 19-4 shows some sources of water contamination.
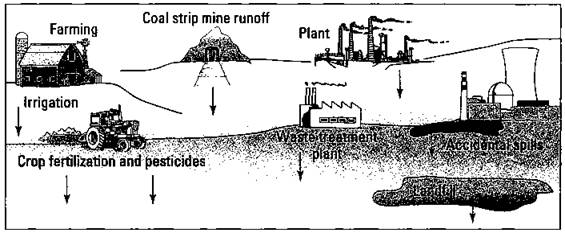
Figure 19-4: Some sources of water pollution.
I call Figure 19-4 Pollution Place, because it shows so many pollution sources in the same place. Naturally, you won’t find this many pollution sites this close together in too many places in the United States.
Pollution sources are normally classified as point sources or non-point sources:
ü Point sources are pollution sites that have a definite identifiable source. Discharges from a chemical industry or raw sewage from wastewater treatment plants are common examples of point-source pollution. Point sources are easy to identify, control, and regulate. The Environmental Protection Agency (EPA) is the governmental agency that regulates point sources.
ü Non-point sources are pollution sources that are rather diffuse in nature. Good examples of this type of pollution are water contamination caused by agricultural runoff or acid rain. Controlling and regulating this type of pollution is much more difficult because you can’t identify a particular company or individual as the polluter. In recent years, federal and state agencies have attempted to address non-point source pollution. The Clean Water Action Plan of 1998 was one such attempt that focused on watersheds and runoffs.
We really didn't yet the lead out: Heavy metal contamination
Water supplies are closely monitored for heavy metals, because they tend to be very toxic. Major sources of heavy metal contamination include landfills, industries, agriculture, mining, and old water distribution systems.
Lead is one type of heavy metal pollutant that has received a lot of press in recent years. Large amounts of lead entered the environment from the use of leaded gasoline: The tetraethyl lead that was used to boost the gasoline’s octane was oxidized in the combustion process, and a large amount of lead was emitted through exhaust systems. Rain runoff carried the lead into streams where it was deposited. Another source of lead was old pipes in municipal buildings and homes. These pipes were commonly joined with a lead solder that then leached lead into the drinking water.
Mercury is released into the aquatic environment from mercury compounds used to treat seeds from fungus and rot. Runoff from fields washes the mercury compounds into the surface water and sometimes into the groundwater supply.
The automobile is also an indirect source of another heavy metal contaminant, chromium. Chromium compounds (such as CrO42-) are used in chrome plating for bumpers and grills. This plating also requires the use of the cyanide ion (CN-), another major pollutant. These contaminants used to be discharged directly into streams, but now they’re either pretreated to reduce to a less- toxic form or precipitated (formed into a solid) and disposed of in landfills.
Mining also adds to the heavy metal pollution problem. As the earth is mined, deposits of minerals, which contain metals, are exposed. If the chemicals used in extracting ore or coal deposits are acidic, then the metals in the minerals are dissolved, and they may make their way into the surface water and sometimes the groundwater. This problem is sometimes controlled with a process that isolates mine drainage and then treats it to remove the metal ions.
Biological concentration is a problem that occurs when industries release heavy metal ions into the waterways. As metal ions move through the ecosystem, they become more and more concentrated. (The same thing happens with radioisotopes — see Chapter 5 for details.) The ions may be released at a very low concentration level, but by the time they move up the food chain to us, the concentration may be at the toxic level. This situation happened in Minamata Bay, Japan. An industry was dumping mercury metal into the bay. As the metal moved through the ecosystem, it was eventually converted to the extremely toxic methylmercury compound. People died as a result of the toxins, and others became permanently affected.
Acid rain
Oxides of nitrogen and sulfur can combine with the moisture in the atmosphere to form rain that can be highly acidic — acid rain. This rain can affect the pH of lakes and streams and has been known to seriously affect aquatic life. In fact, it’s made some lakes devoid of life altogether.
Acid rain is a good example of a non-point source of pollution. It’s difficult to pinpoint a single entity as the cause. Air pollution controls have decreased the amount of acid rain produced, but it’s still a major problem. (If you’d like more info on acid rain, flip to Chapter 18.)
Infectious agents
This category of contamination includes fecal coliform bacteria from human wastes and the wastes of birds and other animals. Fecal coliform bacteria was once a major problem in the United States and most parts of the world. Epidemics of typhoid, cholera, and dysentery were common. Treatment of wastewater has minimized this problem in industrialized nations, but it’s still a definite problem in underdeveloped nations.
Many experts think that more than three-quarters of the sicknesses in the world are related to biological water contaminates. And even now in the United States, beaches and lakes are still closed at times because of biological contamination.
Stricter controls on municipal water treatment, septic tanks, and runoff from feedlots will help decrease the biological contamination of our water.
Landfills and LUST
Landfills — both the public and hazardous chemical kind — are a major source of groundwater contamination. The landfills that are constructed today require special liners to prevent hazardous materials from leaching into the groundwater. Monitoring equipment is also required to confirm that the hazardous materials don’t leak from the landfills. However, very few landfills in the United States have liners and monitoring systems.
Many landfills contain VOCs (volatile organic chemicals). This group of chemicals includes benzene and toluene (both carcinogens), chlorinated hydrocarbons, such as carbon tetrachloride, and trichloroethylene, which previously was used as a dry-cleaning solvent. Even though these compounds are not very soluble in water, they do accumulate at the parts-per-million level. Their long-term effect on human health is unknown at this time.
Most people think of toxic wastes in terms of an industrial dump, but the municipal landfill is becoming a more popular site for the disposed of hazardous household wastes. Every year, tons of the following toxic materials are placed in commercial landfills:
ü Batteries containing heavy metals like mercury
ü Oil-based paints containing organic solvents
ü Motor oil containing metals and organic compounds
ü Gasoline containing organic solvents
ü Automobile batteries containing sulfuric acid and lead
ü Antifreeze containing organic solvents
ü Household insecticides containing organic solvents and pesticides
ü Fire and smoke detectors containing radioactive isotopes
ü Nail polish remover containing organic solvents
Some cities and states are trying to reduce the amount of toxic substances released into the environment by providing special collection sites for materials such as used motor oil. But much more needs to be done.
LUST (leaking underground storage tanks) is another source of VOCs. The major culprit? Old, rusted gasoline storage tanks — especially from filling stations that have long been out of business. It takes less than a gallon of leaked gasoline to contaminate the water supply of a mid-sized town. Recent federal regulations have required the identification and replacement of leaking tanks, but abandoned service stations have become a major problem. It’s estimated that as many as 200,000 tanks still need to be replaced.
The problem of hazardous materials in landfills and the contamination of our water supply prompted Congress to pass the Superfund program. This program was designed to identify and clean up potentially harmful landfills and dumps. Some progress has been made, but there may be thousands of dump sites that need to be cleaned at a monumental cost to the taxpayer.
The alternatives to landfills are recycling and incineration. Some of the material that commonly goes into a public landfill can be recycled — paper, glass, aluminum, and some plastics, for example — but more needs to be done. Incineration of some materials can be accomplished with the generation of electricity. Modern incineration produces very little air pollution.
Agricultural water pollution
Many types of water pollution are associated with the agricultural industry. For example, the excessive use of fertilizers, which contain nitrate and phosphate compounds, have caused a dramatic increase in the growth of algae and plants in lakes and streams. This increased growth may interfere with the normal cycles that occur in these aquatic systems, causing them to age prematurely — a process known as eutrophication.
In addition, pesticides used in treating crops may be released into the waterways. These pesticides, especially the organo-phosphorus ones, may undergo biological concentration (see “We really didn’t get the lead out: Heavy metal contamination,” earlier in this chapter). Many of us remember the reported impact of DDT on fish and birds. Because of the effects of DDT, the United States has banned its use, but it’s still manufactured and sold overseas.
The release of soil and silt into the waterways is another form of pollution associated with the agricultural industry. The soil builds up in the water and interferes with the normal cycles of lakes and streams. It also carries agricultural chemicals into the waterways.
Polluting With heat: Thermal pollution
People usually think of things like lead, mercury, toxic organic compounds, and bacteria as being major pollutants. However, heat can also be a major pollutant. The solubility of a gas in a liquid decreases as the temperature increases (see Chapter 13 for more about the solubility of gases). This means that warm water doesn’t contain as much dissolved oxygen as cool water. And how is this related to pollution? The amount of oxygen in water has a direct impact on aquatic life. The reduction of the dissolved oxygen content of water caused by heat is called thermal pollution.
Industries, especially those that generate electric power, use a tremendous amount of water to cool steam and condense it back to water. This water is normally taken from a lake or stream, used in the cooling process, and then returned to the same body of water. If the heated water is returned directly to the lake or stream, the increase in temperature may cause the oxygen levels to decrease below those required for the survival of certain types of fish. The increased temperature may also trigger or repress natural cycles of aquatic life, such as spawning.
Federal regulations prohibit the release of heated water back into lakes or streams. Industries cool the water by allowing it to remain in pools or running it over the outside of cooling towers. The cooling towers help the water release its heat to the atmosphere. Both of these methods, however, lose a lot of water to evaporation. (And believe me, there are some places in the United States that certainly don’t need the increased humidity.)
Using up oxygen — BOD
If organic material (such as raw sewage, organic chemicals, or a dead cow) finds its way into the water, it decays. The decaying process is basically the oxidation of the organic compounds by aerobic bacteria, or oxygen-consuming bacteria, into simpler molecules such as carbon dioxide and water.
The process requires dissolved oxygen (DO) from the water. The amount of oxygen needed to oxidize the organic material is called the biological oxygen demand (BOD), and it’s normally measured in parts per million (ppm) of oxygen needed. If the BOD is too high, too much dissolved oxygen is used, and there’s not enough oxygen remaining for the fish. Fish kills occur, leading to an even higher BOD.
In extreme cases, there’s not enough oxygen for the aerobic bacteria to survive, so another group of bacteria, anaerobic bacteria, assumes the job of decomposing the organic material. Anaerobic bacteria doesn’t use oxygen in the water; instead, it uses oxygen that’s in the organic compounds. Anaerobic bacteria reduces the waste instead of oxidizing it. (See Chapter 9 for a discussion of oxidation and reduction.) The bad news is that anaerobic bacteria decomposes organic matter into foul-smelling compounds such as hydrogen sulfide (H2S), ammonia, and amines.
In order to stop overloading the BOD of the waterways, most chemical industries pretreat (normally with oxidization) their waste chemicals before releasing them into the water. Cities and towns do the same with their wastewater treatment plants.
Wastewater Treatment
The days when towns and cities in the United States could dump raw, untreated sewage into the waterways are largely over. Every once in a while, a treatment plant malfunctions or becomes overloaded due to some natural disaster and has to dump raw sewage, but those situations are few and far between.
It’s not like that in the rest of the world. In South Asia and most of Africa, for example, very little of the sewage gets treated. But in the United States, sewage gets at least primary treatment; it often gets secondary and tertiary treatment, too. Figure 19-5 diagrams both the primary and secondary treatment of sewage.
Primary sewage treatment
In primary sewage treatment, raw sewage basically undergoes settling and filtration. The sewage first goes through a grate and screen system to remove large items. (I don’t even want to talk about what those items are.) It then moves through a grit chamber where more material is filtered. Finally, it goes to a primary sedimentation tank, where the material is treated with solutions of aluminum sulfate and calcium hydroxide. The two solutions form aluminum hydroxide, a gelatinous precipitate (solid) that accumulates dirt and bacteria as it settles. This primary treatment removes about 50 to 75 percent of the solids, but it only reduces the biological oxygen demand (BOD) by about 30 percent.
If primary treatment is all that the wastewater undergoes, then sometimes chlorine is added to kill a majority of the bacteria before the wastewater is returned to the waterways. It still contains a high BOD, though. So if the waterway is a lake or slow-moving stream, then the high BOD causes problems — especially if a number of towns use the same type of sewage treatment. The problems can be prevented with secondary sewage treatment.

Figure 19-5: Primary and secondary sewage treatment.
Secondary sewage treatment
In secondary sewage treatment; bacteria and other microorganisms are given the opportunity to decompose the organic compounds in the wastewater. Because aerobic bacteria (oxygen-consuming bacteria) produces products that are less noxious than those produced by anaerobic bacteria (bacteria that uses oxygen in the organic compounds instead of oxygen in the water), the sewage is commonly aerated in order to provide the needed oxygen.
Both the primary and secondary processes produce a material called sludge, which is a mixture of particulate matter and living and dead microorganisms. The sludge is dried and disposed of by incineration or in a landfill. It can even be spread on certain types of cropland, where it acts as a fertilizer.
But even secondary treatment can’t remove some substances that are potentially harmful to the environment. These substances include certain organic compounds, certain metals such as aluminum, and fertilizers such as phosphates and nitrates. Tertiary sewage treatment can be used to remove these substances.
Tertiary sewage treatment
Tertiary sewage treatment is essentially a chemical treatment that removes the fine particles, nitrates, and phosphates in wastewater. The basic procedure is adjusted for the specific substance to be removed. Activated charcoal filtration, for example, is used to remove most of the dissolved organic compounds. And alum (Al2(SO4)3) is used to precipitate phosphate ions: by dissolving and freeing the aluminum cation.
![]()
Ion exchange (Chapter 9), reverse osmosis (Chapter 11), and distillation (Chapter 15) are also occasionally used with this type of treatment. All these procedures are relatively expensive, though, so tertiary treatment isn’t done unless really necessary.
Even after tertiary treatment is completed, the wastewater must still be disinfected before it’s released back into the waterways. It’s commonly disinfected by bubbling chlorine gas (CIO into the water. Chlorine gas is an extremely powerful oxidizing agent, and it’s very effective at killing the organisms responsible for cholera, dysentery, and typhoid. But the use of chlorine has come under question lately. If residual organic compounds are in the wastewater, they can be converted into chlorinated hydrocarbons. Several of these compounds have been shown to be carcinogenic. The levels of these compounds are being closely monitored during testing of wastewater. Ozone (O3) can also be used to disinfect wastewater. It’s effective at killing viruses that chlorine can’t kill. It’s more expensive, however, and doesn’t provide the residual protection against bacteria.
Drinking Water Treatment
One of the things we tend to take for granted is the availability of good drinking water. Most people of the world aren’t as fortunate as we are.
The water is brought in from a lake, stream, or reservoir and initially filtered to remove sticks, leaves, dead fish, and such. The turbidity (haziness) that’s commonly present in river or lake water is removed through treatment with a mixture of alum (aluminum sulfate) and lime (calcium hydroxide), which forms gelatinous aluminum hydroxide and traps the suspended solids. This is basically the same treatment used in wastewater treatment plants (see “Primary sewage treatment”).
Then the water is filtered again to remove the solid mass of fine particles (called a flocculate or floe) leftover from the initial filtering treatment. Chlorine is added to kill any bacteria in the water. Then it’s run through an activated charcoal filter that absorbs (collects on its surface) and removes substances responsible for taste, odor, and color. Fluoride may be added at this time to help prevent tooth decay. Finally, the purified water is collected in a holding tank, ready for your use.