Chemistry for Dummies
Part IV. Chemistry in Everyday Life: Benefits and Problems
Chapter 18. Cough! Cough! Hack! Hack! Air Pollution
In This Chapter
· Finding out about the parts of the atmosphere involved in air pollution
· Looking at ozone depletion and the Greenhouse Effect
· Examining the causes of photochemical smog and acid rain
This chapter looks at the global problem of air pollution. (I consider the perfume department of a large department store at Christmas time the ultimate in air pollution, but I won’t discuss that here.) I show you the chemical problems involved with air pollution, and I explain how air pollution is linked to modern society and its demand for energy and personal transportation.
Civilization's Effect on the Atmosphere (Or Where This Mess Began)
The air that surrounds the earth — our atmosphere — is absolutely necessary for life. The atmosphere provides oxygen (O2) for respiration and carbon dioxide (CO2) for photosynthesis, the process by which organisms (mainly plants) convert light energy into chemical energy; it moderates the temperature of the earth and plays an active part in many of the cycles that sustain life. The atmosphere is affected by many chemical reactions that take place or exist on earth.
When few humans were on earth, mankind’s effect on the atmosphere was negligible. But as the world’s population grew, the effect of civilization on the atmosphere became increasingly significant. The Industrial Revolution, which gave rise to the construction of large, concentrated industrial sites, added to man’s effect on the atmosphere. As humans burned more fossil fuels — organic substances, such as coal, that are found in underground deposits and used for energy — the amount of carbon dioxide (CO2) and particulates (small, solid particles suspended in the air) in the atmosphere increased significantly. During the Industrial Revolution, humans also began to use more items that released chemical pollutants into the atmosphere, including hairsprays and air conditioners.
The increase in CO2 and particulates, combined with the increase in pollutants, has disrupted delicate balances in the atmosphere. High concentrations of these atmospheric pollutants have led to a multitude of problems such as acid deposition, acidic rain that damages living things, buildings, and statues, and photochemical smog, the brown, irritating haze that often sits over Los Angeles and other cities.
To Breathe or Not to Breathe: Our Atmosphere
The earth’s atmosphere is divided into several layers: the troposphere, the stratosphere, the mesosphere, and the thermosphere. I want to focus on the two layers closest to the earth — the troposphere and stratosphere — because they’re the layers affected the most by humans. They’re also the layers that have the greatest direct effect on human life.
ü The troposphere lies next to the earth and contains the gases we breathe and depend on for survival.
ü The stratosphere contains the ozone layer, which protects us from ultraviolet radiation.
The troposphere: What humans affect most
The troposphere is composed of about 78.1 percent nitrogen (N2), 20.9 percent oxygen (O2), 0.9 percent argon (Ar), 0.03 percent carbon dioxide (CO2), and smaller amounts of various other gases. The troposphere also contains varying amounts of water vapor. These gases are held tight to the earth by the force of gravity. If a balloonist were to rise high into the troposphere, he or she would find the atmospheric gases much thinner due to the decreased pull of gravity on the gases. This effect tells us that the dense layer of gases held tight to the earth is more at risk from the effects of pollution.
The troposphere is the layer where our weather occurs. It’s also the layer that takes the brunt of both natural and man-made pollution because of its proximity to the earth.
Nature pollutes the atmosphere to a certain extent — with noxious hydrogen sulfide (H2S) and particulate matter from volcanoes, and the release of organic compounds from plants such as pine trees. But these pollutants have a minor effect on the troposphere. Humankind, on the other hand, pollutes the troposphere with a large amount of chemicals from automobiles, power plants, and industries. Acid rain and photochemical smog are some of the results of man-made pollutants.
The stratosphere: Protecting humans with the ozone layer
Above the troposphere is the stratosphere, which is where jets and high- altitude balloons fly. The atmosphere is much thinner in this layer because of the decreasing pull of gravity. Few of the heavier pollutants are able to make it to the stratosphere, because gravity holds them tight and close to the surface of the earth. The protective ozone layer resides in the stratosphere; this protective barrier absorbs a large amount of harmful ultraviolet (UV) radiation from the sun and keeps it from reaching the earth.
Even though heavier pollutants don’t make their way to the stratosphere, this layer isn’t immune to the effects of mankind. Some lighter manmade gases do make it into the stratosphere, where they attack the protective ozone layer and destroy it. This destruction can have far-reaching effects on humans because UV radiation is a major cause of skin cancer.
REMEMBER. A chemical substance can be both a good guy and a bad guy. The only difference is where, and in what concentration, it’s found. For example, a person can overdose on water if he or she drinks enough of it. The same goes with the ozone in the stratosphere. On one hand, it shields us from harmful UV radiation. But on the other, it can be an irritant and destroy rubber products (see “Brown Air? (Photochemical Smog)” for details).
Leave My Ozone Atone: Hair Spray, CFCs, and Ozone depletion
The ozone layer absorbs almost 99 percent of the ultraviolet radiation that reaches the earth from the sun. It protects us from the effects of too much ultraviolet radiation, including sunburns, skin cancers, cataracts, and premature aging of the skin. Because of the ozone layer, most of us can enjoy the outdoors without head-to-toe protection.
How is ozone (O3) formed? Well, oxygen in the mesosphere — the part of the earth’s atmosphere between the stratosphere and the thermosphere (the layer that extends to outer space) — is broken apart by ultraviolet radiation into highly reactive oxygen atoms. These oxygen atoms combine with oxygen molecules in the stratosphere to form ozone.
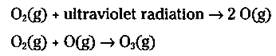
As a society, humans release many gaseous chemicals into the atmosphere. Many of the gaseous chemicals rapidly decompose through reaction with each other, or they react with the water vapor in the atmosphere to form compounds such as acids that fall to earth in the rain (see “Tm Meltingggggg!’ — Acid Rain,” later in this chapter). Besides forming acid rain, some of these chemicals also form photochemical smog (see “Brown Air? (Photochemical Smog),” later in this chapter).
But these reactions occur rather quickly, and we can deal with them in a variety of ways, many of which are related to breaking the series of reactions that produce the pollutant by stopping the release of a critical chemical into the air.
Some classes of gaseous chemical compounds are rather inert (inactive and unreactive), so they remain with us for quite a while. Because these inert compounds stick around, they have a negative effect on the atmosphere. One such troublesome class of compounds are the chlorofluorocarbons, gaseous compounds composed of chlorine, fluorine, and carbon. These compounds are commonly called CFCs.
Because CFCs are relatively unreactive, they were extensively used in the past as refrigerants for such items as refrigerators and automobile air conditioners (Freon-12), foaming agents for plastics such as Styrofoam, and propellants for the aerosol cans of such consumer goods as hair spray and deodorants. As a result, they were released into the atmosphere in great quantities. Over the years, the CFCs have diffused into the stratosphere, and they’re now doing damage to it.
How do CFCs hurt the ozone layer?
Although CFCs don’t react much when they’re close to earth — they’re pretty inert — most scientists believe that they react with the ozone in the atmosphere and then harm the ozone layer in the stratosphere.
The reaction occurs in the following way:
1. A typical chlorofluorocarbon, CF2Cl2, reacts with ultraviolet radiation, and a highly reactive chlorine atom is formed.
![]()
2. The reactive chlorine atom reacts with ozone in the stratosphere to produce oxygen gas molecules and chlorine oxide (CIO).
![]()
This is the reaction that destroys the ozone layer. If things stopped here, the problems would actually be minimal.
3. The chlorine oxide (CIO) can then react with another oxygen atom in the stratosphere to produce an oxygen molecule and a chlorine atom; the newly created oxygen molecule and chlorine atom are now available to start the whole ozone-destroying process all over again.
![]()
So one CFC molecule can initiate a process that can destroy many molecules of ozone.
Because they're harmful, are CFCs stilt produced?
The problem of ozone depletion was identified in the 1970s. As a result, the governments of many industrialized nations began to require the reduction of the amount of CFCs and halons released into the atmosphere. (Halons, which contain bromine in addition to fluorine and chlorine, were commonly used as fire-extinguishing agents, especially in fire extinguishers used around computers.)
CFCs were banned for use as propellants in aerosol cans in many countries, and the CFCs used in the production of plastics and foams were recovered instead of released into the air. Laws were enacted to ensure that the CFCs and halons used as refrigerants were recovered during the recharging and repair of units. In 1991, Du Pont started producing refrigerants that weren’t harmful to the ozone layer. And in 1996, the United States, along with 140 other countries, stopped producing chlorofluorocarbons altogether.
Unfortunately, though, these compounds are extremely stable. They’ll remain in our atmosphere for many years. If the damage man has done to the ozone layer isn’t too great, it may replenish itself (like new skin grows to replace sunburned skin). But it may well be several years before the ozone layer returns to its former composition.
Is It Hot in Here to You? (The Greenhouse Effect)
When most people think about air pollutants, they think of such chemicals as carbon monoxide, chlorofluorocarbons, or hydrocarbons. Yet carbon dioxide, the product of animal respiration and the compound used by plants in the process of photosynthesis, can also be considered a pollutant if present in abnormally high amounts.
In the late 1970s and early 1980s, scientists realized that the average temperature of the earth was increasing. They determined that an increase in carbon dioxide (CO2) and a few other gases, such as chlorofluorocarbons (CFCs), methane (CH4, a hydrocarbon), and water vapor (H2O), were responsible for the slight increase in temperature through a process called the greenhouse effect (named so because the gases serve pretty much the same purpose as the glass walls and roof of a greenhouse — the gases themselves are called greenhouse gases).
Here’s how the greenhouse effect works: As radiation from the sun travels through the earth’s atmosphere, it strikes the earth, heating the land and water. Some of this solar energy is sent back (reflected) into the atmosphere as heat (infrared radiation), which is then absorbed by certain gases (CO2, CH4, H2O, and CFCs) in the atmosphere. These gases, in turn, warm the atmosphere. This process helps to keep the temperature of the earth and atmosphere moderate and relatively constant, and as a result, we don’t experience dramatic day-to-day temperature fluctuations. So, in general, the greenhouse effect is a good thing, not a bad thing.
But if there’s an excess of carbon dioxide and other greenhouse gases, too much heat gets trapped in the atmosphere. The atmosphere heats up, leading to the disruption of many of the delicate cycles of the earth. This process is commonly called global warming, and it’s currently happening with the earth’s atmosphere.
We depend on burning fossil fuels (coal, natural gas, or petroleum) for energy. We burn coal and natural gas to produce electricity, we burn gasoline in the internal combustion engine, and we burn natural gas, oil, wood, and coal to heat homes. In addition, industrial processes burn fuel to produce heat. As a result of all this burning of fossil fuels, the carbon dioxide level in the atmosphere has risen from 318 parts-per-million (ppm) in 1960 to 362 parts- per-million (ppm) in 1998. (For a discussion of the concentration unit ppm, see Chapter 11.) The excess carbon dioxide has led to an increase of about a half-degree in the average temperature of the atmosphere.
A half-degree increase in the average temperature of the atmosphere may not sound like much, but this global warming trend may have serious effects on several of the ecological systems of the world:
ü The rising atmospheric temperature may melt ice masses and cause sea levels around the world to rise. Rising sea levels may result in the loss of coastal land (Houston might become a coastal town) and make many more people vulnerable to storm surges (those extremely damaging rushes of seawater that occur during very bad storms).
ü The increased temperature may affect the growth patterns of plants.
ü The tropical regions of the world may increase and lead to the spread of tropical diseases.
Brown Air? (Photochemical Smog)
Smog is a generic word people use to describe the combination of smoke and fog that’s often irritating to breathe. There are two major types of smog:
ü London smog
ü Photochemical smog
London smog
London smog is a gaseous atmospheric mixture of fog, soot, ash, sulfuric acid (H2SO4 — battery acid), and sulfur dioxide (SO2). The name comes from the air pollution that plagued London in the early part of the twentieth century. The burning of coal for heat in the highly populated city caused this smog. The dangerous mixture of gases and soot from the coal stoves and furnaces killed more than 8,000 people in London in 1952.
Electrostatic precipitators and scrubbers (see “Charge them up and drop them out: Electrostatic precipitators” and “Washing water: Scrubbers,” later in this chapter), combined with filters, have been effective in reducing the release of soot, ash, and sulfur dioxide into the atmosphere and have reduced the occurrence of London smog.
Photochemical smog
Photochemical smog is produced after sunlight initiates certain chemical reactions involving unbumed hydrocarbons and oxides of nitrogen (commonly shown as NOx — which stands for a mixture of NO and NO2). The common automobile engine produces both of these compounds when it’s running.
Photochemical smog is the brown haze that sometimes makes it difficult to see in such cities as Los Angeles, Salt Lake City, Denver, and Phoenix. (This smog is sometimes called Los Angeles smog — sometimes sunny California isn’t so sunny.) These cities are especially vulnerable to photochemical smog; they have a large number of automobiles, which emit the chemicals that react to produce the smog, and they’re surrounded by mountain ranges. The mountain ranges and the westward winds create an ideal condition for thermal inversions, which trap the pollutants close to the cities. (In a thermal inversion, a layer of warmer air moves in over a layer of cooler air. The warm air traps the cooler air and its pollutants close to the ground. The process can be compared to sheets trapping certain noxious gases in a bed. The gaseous pollutants are trapped and can’t move higher in the atmosphere. They stay close to us humans, causing all kinds of problems.)
The chemistry of photochemical smog is still not crystal clear (pun intended), but scientists do know the basics that go into creating the smog. Nitrogen from the atmosphere is oxidized to nitric oxide in internal combustion engines and then released into the atmosphere through the engines’ exhaust systems:
![]()
The nitric oxide is oxidized to nitrogen dioxide by atmospheric oxygen:
![]()
Nitrogen dioxide is a brownish gas. It’s irritating to the eyes and lungs. It absorbs sunlight and then produces nitric oxide and highly reactive oxygen atoms:
![]()
These reactive oxygen atoms quickly react with diatomic (two-atom) oxygen gas molecules in the air to produce ozone (O3):
![]()
This is the same ozone that acts as a shield against ultraviolet radiation in the stratosphere. But when it’s down closer to the earth, it acts as a powerful irritant to the eyes and lungs. It attacks rubber, causing it to harden, and thus shortens the life of automobile tires and weather stripping. It also affects crops such as tomatoes and tobacco.
The unburned hydrocarbons from auto exhaust also react with the oxygen atoms and ozone to produce a variety of organic aldehydes that are also irritants. These hydrocarbons can react with diatomic oxygen and nitrogen dioxide to produce peroxyacetylnitrates (PANs):
![]()
These PANs are also eye and lung irritants; they tend to be very reactive, causing damage to living organisms.
The combination of the brown nitrogen dioxide, the ozone, and the PANs is photochemical smog. It reduces visibility and is a major cause of respiratory problems. And, unfortunately, controlling it has been difficult.
Auto emissions have been closely monitored, and strict controls have been put into place to minimize the amount of unburned hydrocarbons released into the atmosphere. The Clean Air Act of 1990 was passed to help reduce hydrocarbon emissions from automobiles. The catalytic converter was developed to help react the unburned hydrocarbon and produce a less dangerous emission of carbon dioxide and water. (As a side benefit, lead had to be eliminated from gasoline because it “poisoned” the catalyst and made the catalytic converter useless. The big campaign to “get the lead out” removed a major source of the deadly heavy metal from the environment.)
Although such measures as catalytic converters and activated carbon canisters, which are used to help reduce gasoline fumes, have been somewhat effective, photochemical smog still presents a problem. Until mankind develops an acceptable substitute for the internal combustion engine or requires mass transit, photochemical smog will remain with us for years to come.
“I’m Meltinqqqqqq!" — Acid Rain
The wicked witch in The Wizard of Oz dissolved in water. Sometimes buildings do the same because of the action of acid rain on the limestone and marble.
Rainwater is naturally acidic (with a pH less than 7) as a result of the dissolving of carbon dioxide in the moisture of the atmosphere and the forming of carbonic acid. (See Chapter 12 for more about carbonic acid as well as the pH scale.) This interaction results in rainwater having a pH of around 5.6. The term acid rain, or acid deposition, is used to describe a situation in which rainfall has a much lower (more acidic) pH than can be explained by the simple dissolving of carbon dioxide. Specifically, acid rain is formed when certain pollutants in the atmosphere, primarily oxides of nitrogen and sulfur, dissolve in the moisture of the atmosphere and fall to earth as rain with a low pH value.
Oxides of nitrogen (NO, NO2, and so on) are produced naturally during lightning discharges in the atmosphere. This is one way that nature “fixes” nitrogen, or puts it in a form that can be used by plants. However, man adds tremendously to the local amount of atmospheric nitrogen oxides through the use of automobiles. The internal combustion engine reacts the gasoline hydrocarbons with the oxygen in the air, producing carbon dioxide (and carbon monoxide) and water. But the nitrogen that’s present in the air (about 78 percent of the air is nitrogen) may also react with the oxygen at the high temperatures present in the engine. This can produce nitric oxide (NO), which is then released into the atmosphere:
![]()
As the NO enters the atmosphere, it reacts with additional oxygen gas to produce nitrogen dioxide (NO2):
![]()
This nitrogen dioxide can then react with the water vapor in the atmosphere to form nitric and nitrous acids:
![]()
These dilute acid solutions fall to earth as rain with a low pH value — generally in the 4.0 to 4.5 range (although rains with a pH as low as 1.5 have been reported).
A significant amount of acid rain in the eastern part of the United States is caused by oxides of nitrogen, but the acid rain of the Midwest and West is caused by mostly oxides of sulfur, which are primarily generated by power plants and the burning of coal and oil. Sulfur-containing compounds are found as impurities in coal and oil, sometimes as high as 4 percent by weight. These compounds, when burned, produce a sulfur dioxide gas (SO2). Many millions of tons of SO2 are released into the atmosphere each year from power-generating plants. The SO2 reacts with the water vapor in the atmosphere to produce sulfurous acid (H2SO3), and with the oxygen in the atmosphere to produce sulfur trioxide (SO3):
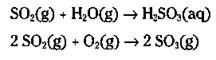
This sulfur trioxide then reacts with the moisture in the atmosphere to produce sulfuric acid (H2SO4), which is the same acid found in your automobile battery:
![]()
So the sulfurous and sulfuric acids that are dissolved in the rainwater form the acid rain that falls to the earth. Anyone for a bath in battery acid?
The acids formed in the atmosphere can travel many hundreds of miles before falling to earth as acid rain and leaving their mark on both nonliving and living things. The acids of the rain react with the iron in buildings and automobiles, causing them to corrode. The acids also destroy the details of fine works of art when they react with marble statues and limestone buildings to form soluble compounds that wash away. (Want to see this in action? Put a drop of vinegar, an acid, on a piece of marble and then watch the bubbles form as the acid dissolves the marble. Careful, though. Don’t try this on anything too valuable — maybe that ugly marble cheese sheer that Aunt Gertrude gave you last Christmas.)
It’s not surprising that acid rain has a bad effect on vegetation. Acid rain has been identified as the major cause of death of many trees and even whole forests. Even if trees aren’t killed immediately by acid rain, forests sometimes grow slower because of its effects. The growth may be hindered by the release of aluminum from the soil, which interferes with the absorption of nutrients, or it may be slowed by the bacteria found in the soil.
In addition, acid rain has altered the ecosystems of many lakes in Canada and the United States. Fish kills have been reported, and entire species of fish have vanished from certain lakes. In fact, the ecosystems of entire lakes have been destroyed by acid rain, rendering the lakes lifeless.
Steps have been taken to reduce acid rain and its effects. Increasing fuel efficiency and the use of pollution control devices on automobiles have helped reduce the amount of nitrogen oxide released into the atmosphere. But fossil fuel power plants produce the most tonnage of acid-causing pollutants. A number of controls have been adopted to decrease the amount of sulfur-containing gases released into the atmosphere, including electrostatic precipitators and scrubbers, which are discussed in the following two sections. But although they’ve been effective in reducing the amount of acid-causing material released into the atmosphere, much more still needs to be done before the problem of acid rain is reduced to a manageable level.
Charge them up and drop them out: Electrostatic precipitators
When you were a child, did you ever run a comb through your hair on a cold winter morning and then use it to pick up little scraps of paper? An electrostatic precipitator does much the same thing.
Electrostatic precipitators give a negative electrical charge to pollutant particles. The sides of the precipitator have a positive charge, so the negative particles are then pulled to the positively charged walls. They stick to the walls and accumulate there. Then they can be removed (it’s like sweeping out those dust bunnies from under the bed).
In one type of electrostatic precipitation system, the SO2 produced by the burning of fossil fuels is reacted with lime (CaO) to produce solid calcium sulfite (CaSO3):
![]()
The finely divided calcium sulfite is electrostatically precipitated and collected. It can then be disposed of properly in a chemical landfill.
Washing water: Scrubbers
Scrubbers are thingies that remove impurities from pollutant gases by using a fine spray of water to trap the gases as an aqueous solution or force them through a reacting mixture. The process is similar to using a water spray to settle dust in arid regions.
You can use a scrubber as an especially efficient system for removing sulfur dioxide by forcing the SO2 through a slurry of magnesium hydroxide and converting it to magnesium sulfite, which can then easily be collected:
![]()
Is the quality of air getting better?
The quality of air in cities such as Los Angeles has improved over the last 15 years. Pollution controls have reduced the oxides of nitrogen and unburned hydrocarbons released by automobiles, and the levels of photochemical smog have been significantly reduced.
Pollution controls have also reduced the levels of sulfur dioxides released from power plants, which has helped lower the occurrence of acid rain. In addition, the ban on CFCs released into the atmosphere should eventually have an effect on ozone depletion. So in many respects, yes, the quality of our air is improving.
But humans are still releasing tremendous amounts of carbon dioxide into the atmosphere and using up large amounts of the earth's valuable plant and animal material (biomass). It is this biomass that really would tend to use up this excess carbon dioxide.
The effect on the environment is debated on a daily basis. All agree that the effect is negative. The question is simply a matter of degree, if mankind can reduce its dependence on fossil fuels for electricity and heat by using solar, nuclear, of perhaps even fusion power, then maybe we'll be able to make advances in reducing the amount of carbon dioxide released into the atmosphere. This strategy, combined with limits on the destruction of forests, may bring the problem of global warming under control.