Chemistry for Dummies
Part II. Blessed Be the Bonds That Tie
In this part...
Mention chemistry, and most people immediately www think of chemical reactions. Scientists use chemical reactions to make new drugs, plastics, cleaners, fabrics — the list is endless. They also use chemical reactions to analyze samples and find out what and how much is in them. Chemical reactions power our bodies, our sun, and our universe. Chemistry is all reactions and the bonding that occurs in them. And that’s what this part is all about.
These chapters introduce you to the two main types of bonding found in nature: ionic bonding and covalent bonding. I show you how to predict the formulas of ionic compounds (salts) and how to name them. I explain covalent bonding, how to draw Lewis structural formulas, and how to predict the shapes of simple molecules. I tell you about chemical reactions and show you the various general types. In addition, I cover chemical equilibrium, kinetics, and electrochemistry — batteries, cells, and electroplating.
I think you’ll get a charge out of the material in this part. In fact, I don’t see how you can fail to react to it.
Chapter 6. Opposites Do Attract Ionic Bonds
In This Chapter
· Finding out why and how ions are formed
· Discovering how cations and ions are formed
· Understanding polyatomic ions
· Deciphering the formulas of ionic compounds
· Naming ionic compounds
· Clarifying the difference between electrolytes and nonelectrolytes
If I had to point to the one thing that made me want to major in chemistry, it would be the reactions of salts. I remember the day clearly: It was the second half of general chemistry, and I was doing qualitative analysis (finding out what’s in a sample) of salts. I really enjoyed the colors of the compounds formed in the reactions I was doing, and the labs were fun and challenging. I was hooked.
In this chapter, I introduce you to ionic bonding, the type of bonding that holds salts together. I discuss simple ions and polyatomic ions: how they form and how they combine. I also show you how to predict the formulas of ionic compounds and how chemists detect ionic bonds.
The Magic of an Ionic Bond: Sodium + Chlorine = Table Salt
Sodium is a fairly typical metal. It’s silvery, soft, and a good conductor. It’s also highly reactive: Sodium is normally stored under oil to keep it from reacting with the water in the atmosphere. If you melt a freshly cut piece of sodium and put it into a beaker filled with greenish-yellow chlorine gas, something very impressive happens. The molten sodium begins to glow with a white light that gets brighter and brighter. The chlorine gas swirls, and soon the color of the gas begins to disappear. In a couple of minutes, the reaction is over, and the beaker can be safely uncovered. You find table salt, or NaCl, deposited on the inside of the beaker.
Understanding the components
If you really stop and think about it, the process of creating table salt is pretty remarkable. You take two substances that are both very hazardous (chlorine was used by the Germans against Allied troops during World War I), and from them you make a substance that’s necessary for life. In this section, I show you what happens during the chemical reaction to create salt and, more importantly, why it occurs.
Sodium is an alkali metal, a member of the IA family on the periodic table. The Roman numerals at the top of the A families show the number of valence electrons (s and p electrons in the outermost energy level) in the particular element (see Chapter 4 for details). So sodium has 1 valence electron and 11 total electrons because its atomic number is 11.
You can use an energy level diagram to represent the distribution of electrons in an atom. Sodium’s energy level diagram is shown in Figure 6-1. (If energy level diagrams are new to you, check out Chapter 3. There are a number of minor variations that are commonly used in writing energy level diagrams, so don’t worry if the diagrams in Chapter 3 are slightly different than the ones I show you here.)
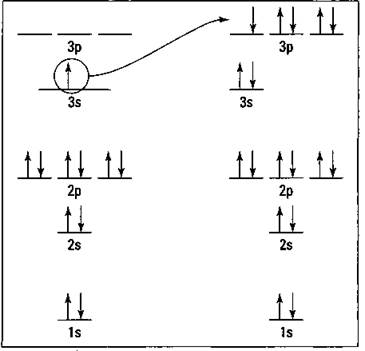
Figure 6-1: Energy level diagram for sodium and chlorine.
Chlorine is a member of the halogen family — the VIIA family on the periodic table. It has 7 valence electrons and a total of 17 electrons. The energy level diagram for chlorine is also shown in Figure 6-1.
If you want, instead of using the bulky energy level diagram to represent the distribution of electrons in an atom, you can use the electron configuration. (For a complete discussion of electron configurations, see Chapter 3.) Write, in order, the energy levels being used, the orbital types (s, p, d, and so on), and — in superscript — the number of electrons in each orbital. Here are the electronic configurations for sodium and chlorine:
Sodium (Na) 1s22s22p63s1
Chlorine (Cl) 1s22s22p63s23p5
Understanding the reaction
The noble gases are the VIIIA elements on the periodic table. They’re extremely unreactive because their valence energy level (outermost energy level) is filled. Achieving a filled (complete) valence energy level is a driving force in nature in terms of chemical reactions, because that’s when elements become stable, or “satisfied.” They don’t lose, gain, or share electrons.
The other elements in the A families on the periodic table do gain, lose, or share valence electrons in order to fill their valence energy level and become satisfied. Because this process, in most cases, involves filling the outermost s and p orbitals, it’s sometimes called the octet rule — elements gain, lose, or share electrons to reach a full octet (8 valence electrons: 2 in the s orbital and 6 in the p orbital).
Sodium's rote
Sodium has one valence electron; by the octet rule, it becomes stable when it has eight valence electrons. Two possibilities exist for sodium to become stable: It can gain seven more electrons to fill energy level 3, or it can lose the one 3s electron so that energy level 2 (which is filled at eight electrons) becomes the valence energy level. In general, the loss or gain of one, two, or sometimes even three electrons can occur, but an element doesn’t lose or gain more than three electrons. So to gain stability, sodium loses its 3s electron. At this point, it has 11 protons (11 positive charges) and 10 electrons (10 negative charges). The once neutral sodium atom now has a single positive charge [11(+) plus 10(-) equals 1+]. It’s now an ion, an atom that has a charge due to the loss or gain of electrons. And ions that have a positive charge (such as sodium) due to the loss of electrons are called cations. You can write an electron configuration for the sodium cation:
Na+ 1s22s22p6
The sodium ion (cation) has the same electron configuration as neon, so it’s isoelectronic with neon. So has sodium become neon by losing an electron? No. Sodium still has 11 protons, and the number of protons determines the identity of the element.
There’s a difference between the neutral sodium atom and the sodium cation — one electron. In addition, their chemical reactivities are different and their sizes are different. The cation is smaller. The filled energy level determines the size of an atom or ion (or, in this case, cation). Because sodium loses an entire energy level to change from an atom to a cation, the cation is smaller.
Chlorine's rote
Chlorine has seven valence electrons. To obtain its full octet, it must lose the seven electrons in energy level 3 or gain one at that level. Because elements don’t gain or lose more than three electrons, chlorine must gain a single electron to fill energy level 3. At this point, chlorine has 17 protons (17 positive charges) and 18 electrons (18 negative charges). So chlorine becomes an ion with a single negative charge (Cl). The neutral chlorine atom becomes the chloride ion. Ions with a negative charge due to the gain of electrons are called anions. The electronic configuration for the chloride anion is
Cl - 1s22s22p63s23p6
The chloride anion is isoelectronic with argon. The chloride anion is also slightly larger than the neutral chlorine atom. To complete the octet, the one electron gained went into energy level 3, but now there are 17 protons attracting 18 electrons. The attractive force has been reduced slightly, and the electrons are free to move outward a little, making the anion a little larger. In general, a cation is smaller than its corresponding atom, and an anion is slightly larger.
Ending up with a band
Sodium can achieve its full octet and stability by losing an electron. Chlorine can fill its octet by gaining an electron. If the two are in the same container, then the electron sodium loses can be the same electron chlorine gains. I show this process in Figure 6-1, indicating that the 3s electron in sodium is transferred to the 3p orbital of chlorine.
The transfer of an electron creates ions — cations (positive charge) and anions (negative charge) — and opposite charges attract each other. The Na+ cation attracts the Cl' anion and forms the compound NaCl, or table salt. This is an example of an ionic bond, which is a chemical bond (a strong attractive force that keeps two chemical elements together) that comes from the electrostatic attraction (attraction of opposite charges) between cations and anions.
The compounds that have ionic bonds are commonly called salts. In sodium chloride, a crystal is formed in which each sodium cation is surrounded by six different chloride anions, and each chloride anion is surrounded by six different sodium cations. The crystal structure is shown in Figure 6-2.
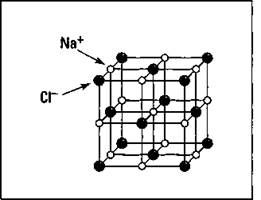
Figure 6-2: Crystal structure of sodium chloride.
Notice the regular, repeating structure. Different types of salts have different crystal structures. Cations and anions can have more than one unit of positive or negative charge if they lose or gain more than one electron. In this fashion, many different kinds of salts are possible.
Ionic bonding, the bonding that holds the cations and anions together in a salt, is one of the two major types of bonding in chemistry. The other type, covalent bonding, is described in Chapter 7. Grasping the concepts involved in ionic bonding makes understanding covalent bonding much easier.
Positive and Negative Ions: Cations and Anions
The basic process that occurs when sodium chloride is formed also occurs when other salts sire formed. A metal loses electrons, and a nonmetal gains those electrons. Cations and anions are formed, and the electrostatic attraction between the positives and negatives brings the particles together and creates the ionic compound.
REMEMBER. A metal reacts with a nonmetal to form an ionic bond.
You can often determine the charge an ion normally has by the element’s position on the periodic table. For example, all the alkali metals (the IA elements) lose a single electron to form a cation with a 1+ charge. In the same way, the alkaline earth metals (IIA elements) lose two electrons to form a 2+ cation. Aluminum, a member of the IIIA family, loses three electrons to form a 3+ cation.
By the same reasoning, the halogens (VIIA elements) all have seven valence electrons. All the halogens gain a single electron to fill their valence energy level. And all of them form an anion with a single negative charge. The VIA elements gain two electrons to form anions with a 2- charge, and the VA elements gain three electrons to form anions with a 3- charge.
Table 6-1 shows the family, element, ion name, and ion symbol for some common monoatomic (one atom) cations, and Table 6-2 gives the same information for some common monoatomic anions.
Table 6-1. Some Common Monoatomic Cations
|
Family |
Element Ion Name Ion Symbol |
||
|
IA |
Lithium |
Lithium cation |
Li+ |
|
|
Sodium |
Sodium cation |
Na+ |
|
|
Potassium |
Potassium cation |
K+ |
|
IIA |
Beryllium |
Beryllium cation |
Be2+ |
|
|
Magnesium |
Magnesium cation |
Mg2+ |
|
|
Calcium |
Calcium cation |
Ca2+ |
|
|
Strontium |
Strontium cation |
Sr2+ |
|
|
Barium |
Barium cation |
Ba2+ |
|
IB |
Silver |
Silver cation |
Ag+ |
|
IIB |
Zinc |
Zinc cation |
Zn2+ |
|
IIIA |
Aluminum |
Aluminum cation |
Al3+ |
Table 6-2. Some Common Monoatomic Anions
|
Family |
Element |
Ion Name |
Ion Symbol |
|
VA |
Nitrogen |
Nitride anion |
N3- |
|
|
Phosphorus |
Phosphide anion |
P3- |
|
VIA |
Oxygen |
Oxide anion |
O2- |
|
|
Sulfur |
Sulfide anion |
S2- |
|
VIIA |
Fluorine |
Fluoride anion |
F- |
|
|
Chlorine |
Chloride anion |
Cl- |
|
|
Bromine |
Bromide anion |
Br- |
|
|
Iodine |
Iodide anion |
I- |
It’s more difficult to determine the number of electrons that members of the transition metals (the B families) lose. In fact, many of these elements lose a varying number of electrons so that they form two or more cations with different charges.
The electrical charge that an atom achieves is sometimes called its oxidation state. Many of the transition metal ions have varying oxidation states. Table 6-3 shows some common transition metals that have more than one oxidation state.
Table 6-3. Some Common Metals with More than One Oxidation State
|
Family |
Element |
Ion Name |
Ion Symbol |
|
VIB |
Chromium |
Chromium(II) or chromous |
Cr2+ |
|
|
|
Chromium(III) or chromic |
Cr3+ |
|
VIIB |
Manganese |
Manganese(II) or manganous |
Mn2+ |
|
|
|
Manganese(III) or manganic |
Mn3+ |
|
VIIIB |
Iron |
Iron(II) or ferrous |
Fe2+ |
|
|
|
Iron(III) or ferric |
Fe3+ |
|
|
Cobalt |
Cobalt(II) or cobaltous |
Co2+ |
|
|
|
Cobalt(III) or cobaltic |
Co3+ |
|
IB |
Copper |
Copper(I) or cuprous |
Cu+ |
|
|
|
Copper(II) or cupric |
Cu2+ |
|
IIB |
Mercury |
Mercury(I) or mercurous |
Hg22+ |
|
|
|
Mercury(II) or mercuric |
Hg2+ |
|
IVA |
Tin |
Tin(II) or stannous |
Sn2+ |
|
|
|
Tin(IV) or stannic |
Sn4+ |
|
|
Lead |
Lead(II)or plumbous |
Pb2+ |
|
|
|
Lead(IV) or plumbic |
Pb4+ |
Notice that these cations can have more than one name. The current way of naming ions is to use the metal name, such as Chromium, followed in parentheses by the ionic charge written as a Roman numeral, such as (II). An older way of naming ions uses -ous and -ic endings. When an element has more than one ion — Chromium, for example — the ion with the lower oxidation state (lower numerical charge, ignoring the + or -) is given an -ous ending, and the ion with the higher oxidation state (higher numerical charge) is given an -ic ending. So for Chromium, the Cr2+ ion is named chromous and the Cr3+ ion is named chromic. (See the section “Naming Ionic Compounds,” later in this chapter, for more on naming ions.)
Polyatomic Ions
Ions aren’t always monoatomic, composed of just one atom. Ions can also be polyatomic, composed of a group of atoms. For example, take a look at Table 6-3. Notice anything about the Mercury(I) ion? Its ion symbol, Hg22+, shows that two mercury atoms are bonded together. This group has a 2+ charge, with each mercury cation having a 1+ charge. The mercurous ion is classified as a polyatomic ion.
Polyatomic ions are treated the same as monoatomic ions (see “Naming Ionic Compounds,” later in this chapter). Table 6-4 lists some important polyatomic ions.
Table 6-4. Some Important Polyatomic Ions
|
Ion Name |
Ion Symbol |
|
|
Sulfate |
SO42- |
|
|
Sulfite |
SO32- |
|
|
Nitrate |
NO3- |
|
|
Nitrite |
NO2- |
|
|
Hypochlorite |
ClO- |
|
|
Chlorite |
ClO2- |
|
|
Chlorate |
ClO3- |
|
|
Perchlorate |
ClO4- |
|
|
Acetate |
C2H3O2- |
|
|
Chromate |
CrO42- |
|
|
Dichromate |
Cr2O72- |
|
|
Arsenate |
AsO43- |
|
|
Hydrogen phosphate |
HPO42- |
|
|
Dihydrogen phosphate |
H2PO4- |
|
|
Bicarbonate or hydrogen carbonate |
HCO3- |
|
|
Bisulfate or hydrogen sulfate |
HSO4- |
|
|
Mercury (I) |
Hg22+ |
|
|
Ammonium |
NH4+ |
|
|
Phosphate |
PO43- |
|
|
Carbonate |
CO32- |
|
|
Permanganate |
MnO4- |
|
|
Cyanide |
CN- |
|
|
Cyanate |
OCN- |
|
|
Thiocyanate |
SCN- |
|
|
Oxalate |
C2O42- |
|
|
Thiosulfate |
S2O32- |
|
|
Hydroxide |
OH- |
|
|
Arsenite |
AsO33- |
|
|
Peroxide |
O22- |
|
The symbol for the sulfate ion, SO42-, indicates that one sulfur atom and four oxygen atoms are bonded together and that the whole polyatomic ion has two extra electrons.
Putting Ions Together: Ionic Compounds
When an ionic compound is formed, the cation and anion attract each other, resulting in a salt (see “The Magic of an Ionic Bond: Sodium + Chlorine = Table Salt,” earlier in this chapter). An important thing to remember is that the compound must be neutral — have equal numbers of positive and negative charges.
Putting magnesium anti bromine together
Suppose you want to know the formula, or composition, of the compound that results from reacting magnesium with bromine. You start by putting the two atoms side by side, with the metal on the left, and then adding their charges. Figure 6-3 shows this process. (Forget about the crisscrossing lines for now. Well, if you’re really curious, they’re discussed in the “Using the crisscross rule” section, later in this chapter.)
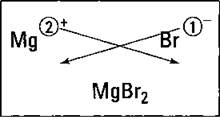
Figure 6-3: Figuring the formula of magnesium bromide.
The electron configurations for magnesium and bromine are
Magnesium (Mg) 1s22s22p63s2
Bromine (Br) 1s22s22p63s23p64s23d104p5
Magnesium, an alkaline earth metal, has two valence electrons that it loses to form a cation with a 2+ charge. The electron configuration for the magnesium cation is
Mg2+ 1s22s22p6
Bromine, a halogen, has seven valence electrons, so it gains one to complete its octet (eight valence electrons) and form the bromide anion with a 1- charge. The electron configuration for the bromide anion is
Br1- 1s22s22p63s23p64s23d104p6
Note that if the anion simply has 1 unit of charge, positive or negative, you normally don’t write the 1; you just use the plus or minus symbol, with the 1 being understood. But for the example of the bromide ion, I use the 1.
The compound must be neutral; it must have the same number of positive and negative charges so that, overall, it has a zero charge. The magnesium ion has a 2+, so it requires 2 bromide anions, each with a single negative charge, to balance the 2 positive charges of magnesium. So the formula of the compound that results from reacting magnesium with bromine is MgBr2.
Using the crisscross rule
There’s a quick way to determine the formula of an ionic compound: Use the crisscross rule.
Look at Figure 6-3 for an example of using this rule. Take the numerical value of the metal ion’s superscript (forget about the charge symbol) and move it to the bottom right-hand side of the nonmetal’s symbol — as a subscript. Then take the numerical value of the nonmetal’s superscript and make it the subscript of the metal. (Note that if the numerical value is 1, it’s just understood and not shown.) So in this example, you make magnesium’s 2 a subscript of bromine and make bromine’s 1 a subscript of magnesium (but because it’s 1, you don’t show it), and you get the formula MgBr2.
So what happens if you react aluminum and oxygen? Figure 6-4 shows the crisscross rule used for this reaction.
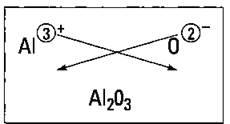
Figure 6-4: Figuring the formula of aluminum oxide.
Compounds involving polyatomic ions work exactly the same way. For example, here’s the compound made from the ammonium cation and the sulfide anion:
(NH4)2S
Notice that because two ammonium ions (two positive charges) are needed to neutralize the two negative charges of the sulfide ion, the ammonium ion is enclosed in parentheses and a subscript 2 is added.
WARNING! The crisscross rule works very well, but there’s a situation where you have to be careful. Suppose that you want to write the compound formed when magnesium reacts with oxygen. Magnesium, an alkaline earth metal, forms a 2+ cation, and oxygen forms a 2- anion. So you might predict that the formula is
Mg2O2
But this formula is incorrect. After you use the crisscross rule, you need to reduce all the subscripts by a common factor, if possible. In this case, you divide each subscript by 2 and get the correct formula:
MgO
Naming Ionic Compounds
When you name inorganic compounds, you write the name of the metal first and then the nonmetal. Suppose, for example, that you want to name Li2S, the compound that results from the reaction of lithium and sulfur. You first write the name of the metal, lithium, and then write the name of the nonmetal, adding an -ide ending so that sulfur becomes sulfide.
Li2S lithium sulfide
Ionic compounds involving polyatomic ions follow the same basic rule: Write the name of the metal first, and then simply add the name of the nonmetal (with the polyatomic anions, it is not necessary to add the -ide ending).
(NH4)2CO3 Ammonium carbonate
K3PO4 Potassium phosphate
When the metal involved is a transition metal with more than one oxidation state (see “Positive and Negative Ions: Cations and Anions,” earlier in the chapter, for more info about that), there can be more than one way to correctly name the compound. For example, suppose that you want to name the compound formed between the Fe3+ cation and the cyanide ion, CN-. The preferred method is to use the metal name followed in parentheses by the ionic charge written as a Roman numeral: Iron(III). But an older naming method, which is still sometimes used (so it’s a good idea to know it), is to use -ous and -ic endings. The ion with the lower oxidation state (lower numerical charge, ignoring the + or -) is given an -ous ending, and the ion with the higher oxidation state (higher numerical charge) is given an -ic ending. So, because Fe3+ has a higher oxidation state than Fe2+, it’s called a ferric ion. So the compound can be named
Fe(CN)3 Iron(III) cyanide or ferric cyanide
Sometimes figuring out the charge on an ion can be a little challenging (and fun), so now I want to show you how to name FeNH4(SO4)2.
I show you in Table 6-4 that the sulfate ion has a 2- charge, and from the formula you can see that there are two of them. Therefore, you have a total of four negative charges. Table 6-4 also indicates that the ammonium ion has a 1+ charge, so you can figure out the charge on the iron cation.
|
Ion |
Charge |
|
Fe |
? |
|
NH4 |
1+ |
|
(SO4)2 |
(2-)x2 |
Because you have a 4- for the sulfates and a 1+ for the ammonium, the iron must be a 3+ to make the compound neutral. So the iron is in the Iron(III), or ferric, oxidation state. You can name the compound
FeNH4(SO4)2 Iron(III) ammonium sulfate or ferric ammonium sulfate
And, finally, if you have the name, you can derive the formula and the charge on the ions. For example, suppose that you’re given the name cuprous oxide. You know that the cuprous ion is Cu+and the oxide ion is O2-. Applying the crisscross rule, you get the following formula:
Cuprous oxide Cu2O
Electrolytes and Nonelectrolytes
When an ionic compound such as sodium chloride is put into water, the water molecules attract both the cations and anions in the crystal (the crystal is shown in Figure 6-2) and pull them into the solution. (In Chapter 7,1 talk a lot about water molecules and show you why they attract the NaCl ions.) The cations and anions get distributed throughout the solution. You can detect the presence of these ions by using an instrument called a conductivity tester.
A conductivity tester tests whether water solutions of various substances conduct electricity. It’s composed of a light bulb with two electrodes attached. The light bulb is plugged into a wall outlet, but it doesn’t light until some type of conductor (substance capable of transmitting electricity) between the electrodes completes the circuit. (A finger will complete the circuit, so this experiment should be done carefully. If you’re not careful, it can be a shocking experience!)
When you place the electrodes in pure water, nothing happens, because there’s no conductor between the electrodes. Pure water is a nonconductor. But if you put the electrodes in the NaCl solution, the light bulb lights, because the ions conduct the electricity (carry the electrons) from one electrode to the other.
In fact, you don’t even really need the water. If you were to melt pure NaCl (it requires a lot of heat!) and then place the electrodes in it, you’d find that the molten table salt also conducts electricity. In the molten state, the NaCl ions are free to move and carry electrons, just as they are in the saltwater solution. Substances that conduct electricity in the molten state or when dissolved in water are called electrolytes. Substances that don’t conduct electricity when in these states are called nonelectrolytes.
Scientists can get some good clues as to the type of bonding in a compound by discovering whether a substance is an electrolyte or a nonelectrolyte. Ionically bonded substances act as electrolytes. But covalently bonded compounds (see Chapter 7), in which no ions are present, are commonly nonelectrolytes. Table sugar, or sucrose, is a good example of a nonelectrolyte. You can dissolve sugar in water or melt it, but it won’t have conductivity. No ions are present to transfer the electrons.