Chemistry for Dummies
Part I. Basic Concepts of Chemistry
Chapter 5. Nuclear Chemistry: If II Blow Your Mind
In This Chapter
· Understanding radioactivity and radioactive decay
· Figuring out half-lives
· The basics of nuclear fission
· Taking a look at nuclear fusion
· Tracing the effects of radiation
Most of this book deals, in one way or the other, with chemical reactions. And when I talk about these reactions, I’m really talking about how the valence electrons (the electrons in the outermost energy levels of atoms) are lost, gained, or shared. I mention very little about the nucleus of the atom because, to a very large degree, it’s not involved in chemical reactions.
But, in this chapter, I do discuss the nucleus and the changes it can undergo. I talk about radioactivity and the different ways an atom can decay. I discuss half-lives and show you why they are important in the storage of nuclear waste products. I also discuss nuclear fission in terms of bombs, power plants, and the hope that nuclear fusion holds for mankind.
Like most of you reading this book, I’m a child of the Atomic Age. I actually remember open air testing of nuclear weapons. I remember being warned not to eat snow because it might contain fallout. I remember friends building fallout shelters. I remember A-bomb drills at school. I remember x-ray machines in shoe stores. (I never did order those x-ray glasses, though!) And I remember radioactive Fiesta stoneware and radium watch hands. When I was growing up, atomic energy was new, exciting, and scary. And it still is.
It All Starts with the Atom
To understand nuclear chemistry, you need to know the basics of atomic structure. Chapter 3 drones on and on (and on) about atomic structure, if you’re interested. This section just provides a quickie brain dump.
The nucleus, that dense central core of the atom, contains both protons and neutrons. Electrons are outside the nucleus in energy levels. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge. A neutral atom contains equal numbers of protons and electrons. But the number of neutrons within an atom of a particular element can vary. Atoms of the same element that have differing numbers of neutrons are called isotopes. Figure 5-1 shows the symbolization chemists use to represent a specific isotope of an element.
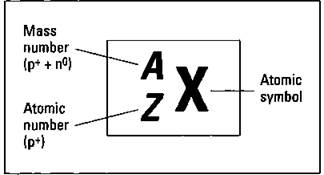
Figure 5-1: Representing a specific isotope.
In the figure, X represents the symbol of the element found on the periodic table, Z represents the atomic number (the number of protons in the nucleus), and A represents the mass number (the sum of the protons and neutrons in that particular isotope). If you subtract the atomic number from the mass number (A - Z), you get the number of neutrons in that particular isotope. A short way to show the same information is to simply use the element symbol (X) and the mass number (A) — for example, U-235.
Radioactivity and Man-Made Radioactive Decay
For purposes of this book, I define radioactivity as the spontaneous decay of an unstable nucleus. An unstable nucleus may break apart into two or more other particles with the release of some energy (see “Gone (Nuclear) Fission,” later in this chapter, for more info on this process). This breaking apart can occur in a number of ways, depending on the particular atom that’s decaying. You can often predict one of the particles of a radioactive decay by knowing the other particle. Doing so involves something called balancing the nuclear reaction. (A nuclear reaction is any reaction involving a change in nuclear structure.)
Balancing a nuclear reaction is really a fairly simple process. But before I explain it, I want to show you how to represent a reaction:
Reactants → Products
Reactants are the substances you start with, and products are the new substances being formed. The arrow, called a reaction arrow, indicates that a reaction has taken place.
For a nuclear reaction to be balanced, the sum of all the atomic numbers on the left-hand side of the reaction arrow must equal the sum of all the atomic numbers on the right-hand side of the arrow. The same is true for the sums of the mass numbers. Here’s an example: Suppose you’re a scientist performing a nuclear reaction by bombarding a particular isotope of chlorine (Cl-35) with a neutron. (Work with me here. I’m just trying to get to a point.) You observe that an isotope of hydrogen, H-1, is created along with another isotope, and you want to figure out what the other isotope is. The equation for this example is
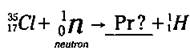
Now to figure out the unknown isotope (represented by Pr), you need to balance the equation. The sum of the atomic numbers on the left is 17 (17 + 0), so you want the sum of the atomic numbers on the right to equal 17 too. Right now, you’ve got an atomic number of 1 on the right; 17 - 1 is 16, so that’s the atomic number of the unknown isotope. This atomic number identifies the element as Sulfur (S).
Now look at the mass numbers in the equation. The sum of the mass numbers on the left is 36 (35 + 1), and you want the sum of the mass numbers on the right to equal 36, too. Right now, you’ve got a mass number of 1 on the right; 36 - 1 is 35, so that’s the mass number of the unknown isotope. Now you know that the unknown isotope is a Sulfur isotope (S-35). And here’s what the balanced nuclear equation looks like:
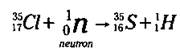
This equation represents a nuclear transmutation, the conversion of one element into another. Nuclear transmutation is a process human beings control. S-35 is an isotope of sulfur that doesn’t exist in nature. It’s a manmade isotope. Alchemists, those ancient predecessors of chemists, dreamed of converting one element into another (usually lead into gold), but they were never able to master the process. Chemists are now able, sometimes, to convert one element into another.
Natural Radioactive Decay: How Nature Does It
Certain isotopes are unstable: Their nucleus breaks apart, undergoing nuclear decay. Sometimes the product of that nuclear decay is unstable itself and undergoes nuclear decay, too. For example, when U-238 (one of the radioactive isotopes of uranium) initially decays, it produces Th-234, which decays to Pa-234. The decay continues until, finally, after a total of 14 steps, Pb-206 is produced. Pb-206 is stable, and the decay sequence, or series, stops.
Before I show you how radioactive isotopes decay, I want to briefly explain why a particular isotope decays. The nucleus has all those positively charged protons shoved together in an extremely small volume of space. All those protons are repelling each other. The forces that normally hold the nucleus together, the “nuclear glue,” sometimes can’t do the job, and so the nucleus breaks apart, undergoing nuclear decay.
All elements with 84 or more protons are unstable; they eventually undergo decay. Other isotopes with fewer protons in their nucleus are also radioactive. The radioactivity corresponds to the neutron/proton ratio in the atom. If the neutron/proton ratio is too high (there are too many neutrons or too few protons), the isotope is said to be neutron rich and is, therefore, unstable. Likewise, if the neutron/proton ratio is too low (there are too few neutrons or too many protons), the isotope is unstable. The neutron/proton ratio for a certain element must fall within a certain range for the element to be stable. That’s why some isotopes of an element are stable and others are radioactive.
There are three primary ways that naturally occurring radioactive isotopes decay:
ü Alpha particle emission
ü Beta particle emission
ü Gamma radiation emission
In addition, there are a couple of less common types of radioactive decay:
ü Positron emission
ü Electron capture
Alpha emission
An alpha particle is defined as a positively charged particle of a helium nuclei. I hear ya: Huh? Try this: An alpha particle is composed of two protons and two neutrons, so it can be represented as a Helium-4 atom. As an alpha particle breaks away from the nucleus of a radioactive atom, it has no electrons, so it has a +2 charge. Therefore and to-wit, it’s a positively charged particle of a helium nuclei. (Well, it’s really a cation, a positively charged ion — see Chapter 3.)
But electrons are basically free — easy to lose and easy to gain. So normally, an alpha particle is shown with no charge because it very rapidly picks up two electrons and becomes a neutral helium atom instead of an ion.
Large, heavy elements, such as uranium and thorium, tend to undergo alpha emission. This decay mode relieves the nucleus of two units of positive charge (two protons) and four units of mass (two protons + two neutrons). What a process. Each time an alpha particle is emitted, four units of mass are lost. I wish I could find a diet that would allow me to lose four pounds at a time!
Radon-222 (Rn-222) is another alpha particle emitter, as shown in the following equation:
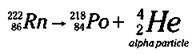
Here, Radon-222 undergoes nuclear decay with the release of an alpha particle. The other remaining isotope must have a mass number of 218 (222 - 4) and an atomic number of 84 (86 - 2), which identifies the element as Polonium (Po). (If this subtraction stuff confuses you, check out how to balance equations in the section “Radioactivity and Man-Made Radioactive Decay,” earlier in this chapter.)
Beta emission
A beta particle is essentially an electron that’s emitted from the nucleus. (Now I know what you’re thinking — electrons aren’t in the nucleus. Keep on reading to find out how they can be formed in this nuclear reaction.) Iodine-131 (1-131), which is used in the detection and treatment of thyroid cancer, is a beta particle emitter:
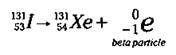
Here, the Iodine-131 gives off a beta particle (an electron), leaving an isotope with a mass number of 131 (131 - 0) and an atomic number of 54 (53 - (-1)). An atomic number of 54 identifies the element as Xenon (Xe).
Notice that the mass number doesn’t change in going from 1-131 to Xe-131, but the atomic number increases by one. In the iodine nucleus, a neutron was converted (decayed) into a proton and an electron, and the electron was emitted from the nucleus as a beta particle. Isotopes with a high neutron/ proton ratio often undergo beta emission, because this decay mode allows the number of neutrons to be decreased by one and the number of protons to be increased by one, thus lowering the neutron/proton ratio.
Gamma emission
Alpha and beta particles have the characteristics of matter: They have definite masses, occupy space, and so on. However, because there is no mass change associated with gamma emission, I refer to gamma emission as gamma radiation emission. Gamma radiation is similar to x-rays — high energy, short wavelength radiation. Gamma radiation commonly accompanies both alpha and beta emission, but it’s usually not shown in a balanced nuclear reaction. Some isotopes, such as Cobalt-60 (Co-60), give off large amounts of gamma radiation. Co-60 is used in the radiation treatment of cancer. The medical personnel focus gamma rays on the tumor, thus destroying it.
Positron emission
Although positron emission doesn’t occur with naturally occurring radioactive isotopes, it does occur naturally in a few man-made ones. A positron is essentially an electron that has a positive charge instead of a negative charge. A positron is formed when a proton in the nucleus decays into a neutron and a positively charged electron. The positron is then emitted from the nucleus. This process occurs in a few isotopes, such as Potassium-40 (K-40), as shown in the following equation:
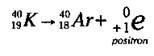
The K-40 emits the positron, leaving an element with a mass number of 40 (40 - 0) and an atomic number of 18 (19 - 1). An isotope of argon (Ar), Ar-40, has been formed.
If you watch Star Trek, you may have heard about antimatter. The positron is a tiny bit of antimatter. When it comes in contact with an electron, both particles are destroyed with the release of energy. Luckily, not many positrons are produced: If a lot of them were produced, you’d probably have to spend a lot of time ducking explosions.
Electron capture
Electron capture is a rare type of nuclear decay in which an electron from the innermost energy level (the Is — see Chapter 3) is captured by the nucleus. This electron combines with a proton to form a neutron. The atomic number decreases by one, but the mass number stays the same. The following equation shows the electron capture of Polonium-204 (Po-204):
![]()
The electron combines with a proton in the polonium nucleus, creating an isotope of bismuth (Bi-204).
The capture of the Is electron leaves a vacancy in the Is orbitals. Electrons drop down to fill the vacancy, releasing energy not in the visible part of the electromagnetic spectrum but in the X-ray portion.
Half-Lives and Radioactive Dating
If you could watch a single atom of a radioactive isotope, U-238, for example, you wouldn’t be able to predict when that particular atom might decay. It might take a millisecond, or it might take a century. There’s simply no way to tell.
But if you have a large enough sample — what mathematicians call a statistically significant sample size — a pattern begins to emerge. It takes a certain amount of time for half the atoms in a sample to decay. It then takes the same amount of time for half the remaining radioactive atoms to decay, and the same amount of time for half of those remaining radioactive atoms to decay, and so on. The amount of time it takes for one-half of a sample to decay is called the half-life of the isotope, and it’s given the symbol t1/2. This process is shown in Table 5-1.
Table 5-1. Half-Life Decay of a Radioactive Isotope
|
Half-Life |
Percent of Radioactive isotope Remaining |
|
0 |
100.00 |
|
1 |
50.00 |
|
2 |
25.00 |
|
3 |
12.50 |
|
4 |
6.25 |
|
5 |
3.12 |
|
6 |
1.56 |
|
7 |
0.78 |
|
8 |
0.39 |
|
9 |
0.19 |
|
10 |
0.09 |
It’s important to realize that the half-life decay of radioactive isotopes is not linear. For example, you can’t find the remaining amount of an isotope as 7.5 half-lives by finding the midpoint between 7 and 8 half-lives. This decay is an example of an exponential decay, shown in Figure 5-2.
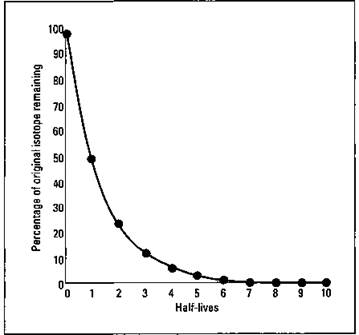
Figure 5-2: Decay of a radioactive isotope.
TECHNICAL STUFF. If you want to find times or amounts that are not associated with a simple multiple of a half-life, you can use this equation:
![]()
In the equation, In stands for the natural logarithm (not the base 10 log; it’s that In button on your calculator, not the log button), N0 is the amount of radioactive isotope that you start with, N is the amount of radioisotope left at some time (t), and t1/2 is the half-life of the radioisotope. If you know the half-life and the amount of the radioactive isotope that you start with, you can use this equation to calculate the amount remaining radioactive at any time. But we are going to keep it simple.
Half-lives may be very short or very long. Table 5-2 shows the half-lives of some typical radioactive isotopes.
Table 5-2. Half-Lives of Some Radioactive Isotopes
|
Radioisotope |
Radiation Emitted |
Half-Life |
|
Kr-94 |
Beta |
1.4 seconds |
|
Rn-222 |
Alpha |
3.8 days |
|
1-131 |
Beta |
8 days |
|
Co-60 |
Gamma |
5.2 years |
|
H-3 |
Beta |
12.3 years |
|
C-14 |
Beta |
5,730 years |
|
U-235 |
Alpha |
4.5 billion years |
|
Re-187 |
Beta |
70 billion years |
Safe handling
Knowing about half-lives is important because it enables you to determine when a sample of radioactive material is safe to handle. The rule is that a sample is safe when its radioactivity has dropped below detection limits. And that occurs at 10 half-lives. So, if radioactive iodine-131 (t1/2 = 8 days) is injected into the body to treat thyroid cancer, it’ll be “gone” in 10 half-lives, or 80 days.
This stuff is important to know when using radioactive isotopes as medical tracers, which are taken into the body to allow doctors to trace a pathway or find a blockage, or in cancer treatments. They need to be active long enough to treat the condition, but they should also have a short enough half-life so that they don’t injure healthy cells and organs.
Radioactive dating
A useful application of half-lives is radioactive dating. No, radioactive dating has nothing to do with taking an X-ray tech to the movies. It has to do with figuring out the age of ancient things.
Carbon-14 (C-14), a radioactive isotope of carbon, is produced in the upper atmosphere by cosmic radiation. The primary carbon-containing compound in the atmosphere is carbon dioxide, and a very small amount of carbon dioxide contains C-14. Plants absorb C-14 during photosynthesis, so C-14 is incorporated into the cellular structure of plants. Plants are then eaten by animals, making C-14 a part of the cellular structure of all living things.
As long as an organism is alive, the amount of C-14 in its cellular structure remains constant. But when the organism dies, the amount of C-14 begins to decrease. Scientists know the half-life of C-14 (5,730 years, listed in Table 5-2), so they can figure out how long ago the organism died.
Radioactive dating using C-14 has been used to determine the age of skeletons found at archeological sites. Recently, it was used to date the Shroud of Turin, a piece of linen in the shape of a burial cloth that contains an image of a man. Many thought that it was the burial cloth of Jesus, but in 1988, radiocarbon dating determined that the cloth dated from around a.d. 1200-1300. Even though we don’t know how the image of the man was placed on the Shroud, C-14 dating has proven that it’s not the death cloth of Jesus.
Carbon-14 dating can only be used to determine the age of something that was once alive. It can’t be used to determine the age of a moon rock or a meteorite. For nonliving substances, scientists use other isotopes, such as potassium-40.
Gone (Nuclear) Fission
In the 1930s, scientists discovered that some nuclear reactions can be initiated and controlled (see “Radioactivity and Man-Made Radioactive Decay,” earlier in this chapter). Scientists usually accomplished this task by bombarding a large isotope with a second, smaller one — commonly a neutron. The collision caused the larger isotope to break apart into two or more elements, which is called nuclear fission. The nuclear fission of uranium-235 is shown in the following equation:
![]()
Reactions of this type also release a lot of energy. Where does the energy come from? Well, if you make very accurate measurement of the masses of all the atoms and subatomic particles you start with and all the atoms and subatomic particles you end up with, and then compare the two, you find that there’s some “missing” mass. Matter disappears during the nuclear reaction. This loss of matter is called the mass defect. The missing matter is converted into energy.
You can actually calculate the amount of energy produced during a nuclear reaction with a fairly simple equation developed by Einstein: E = mc2. In this equation, E is the amount of energy produced, m is the “missing” mass, or the mass defect, and c is the speed of light, which is a rather large number. The speed of light is squared, making that part of the equation a very large number that, even when multiplied by a small amount of mass, yields a large amount of energy.
Chain reactions and critical mass
Take a look at the equation for the fission of U-235 in the preceding section. Notice that one neutron was used, but three were produced. These three neutrons, if they encounter other U-235 atoms, can initiate other fissions, producing even more neutrons. It’s the old domino effect. In terms of nuclear chemistry, it’s a continuing cascade of nuclear fissions called a chain reaction. The chain reaction of U-235 is shown in Figure 5-3.
This chain reaction depends on the release of more neutrons than were used during the nuclear reaction. If you were to write the equation for the nuclear fission of U-238, the more abundant isotope of uranium, you’d use one neutron and only get one back out. You can’t have a chain reaction with U-238. But isotopes that produce an excess of neutrons in their fission support a chain reaction. This type of isotope is said to be fissionable, and there are only two main fissionable isotopes used during nuclear reactions — uranium-235 and plutonium-239.
A certain minimum amount of fissionable matter is needed to support a self-sustaining chain reaction, and it’s related to those neutrons. If the sample is small, then the neutrons are likely to shoot out of the sample before hitting a U-235 nucleus. If they don’t hit a U-235 nucleus, no extra electrons and no energy are released. The reaction just fizzles. The minimum amount of fissionable material needed to ensure that a chain reaction occurs is called the critical mass. Anything less than this amount is called subcritical.
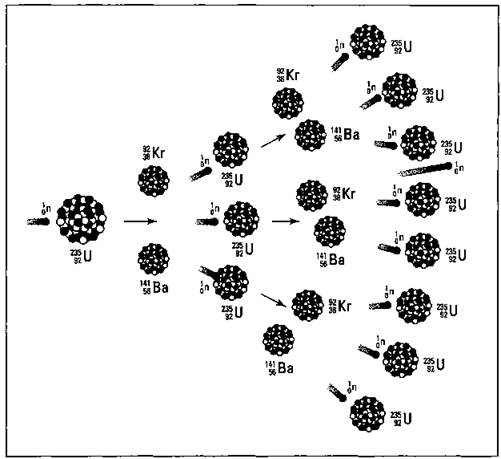
Figure 5-3: Chain reaction.
Atomic bombs (big bangs that aren't theories)
Because of the tremendous amount of energy released in a fission chain reaction, the military implications of nuclear reactions were immediately realized. The first atomic bomb was dropped on Hiroshima, Japan, on August 6,1945.
In an atomic bomb, two pieces of a fissionable isotope are kept apart. Each piece, by itself, is subcritical. When it’s time for the bomb to explode, conventional explosives force the two pieces together to cause a critical mass. The chain reaction is uncontrolled, releasing a tremendous amount of energy almost instantaneously.
The real trick, however, is to control the chain reaction, releasing its energy slowly so that ends other than destruction might be achieved.
Nuclear pouter plants
The secret to controlling a chain reaction is to control the neutrons. If the neutrons can be controlled, then the energy can be released in a controlled way. That’s what scientists have done with nuclear power plants.
In many respects, a nuclear power plant is similar to a conventional fossil fuel power plant. In this type of plant, a fossil fuel (coal, oil, natural gas) is burned, and the heat is used to boil water, which, in turn, is used to make steam. The steam is then used to turn a turbine that is attached to a generator that produces electricity.
The big difference between a conventional power plant and a nuclear power plant is that the nuclear power plant produces heat through nuclear fission chain reactions.
How do nuclear pouter plants make electricity?
Most people believe that the concepts behind nuclear power plants are tremendously complex. That’s really not the case. Nuclear power plants are very similar to conventional fossil fuel plants.
The fissionable isotope is contained in fuel rods in the reactor core. All the fuel rods together comprise the critical mass. Control rods, commonly made of boron or cadmium, are in the core, and they act like neutron sponges to control the rate of radioactive decay. Operators can stop a chain reaction completely by pushing the control rods all the way into the reactor core, where they absorb all the neutrons. The operators can then pull out the control rods a little at a time to produce the desired amount of heat.
A liquid (water or, sometimes, liquid sodium) is circulated through the reactor core, and the heat generated by the fission reaction is absorbed. The liquid then flows into a steam generator, where steam is produced as the heat is absorbed by water. This steam is then piped through a steam turbine that’s connected to an electric generator. The steam is condensed and recycled through the steam generator. This forms a closed system; that is, no water or steam escapes — it’s all recycled.
The liquid that circulates through the reactor core is also part of a closed system. This closed system helps ensure that no contamination of the air or water takes place. But sometimes problems do arise.
Oh, so many problems
In the United States, there are approximately 100 nuclear reactors, producing a little more than 20 percent of the country’s electricity. In France, almost 80 percent of the country’s electricity is generated through nuclear fission. Nuclear power plants have certain advantages. No fossil fuels are burned (saving fossil-fuel resources for producing plastics and medicines), and there are no combustion products, such as carbon dioxide, sulfur dioxide, and so on, to pollute the air and water. But problems are associated with nuclear power plants.
One is cost. Nuclear power plants are expensive to build and operate. The electricity that’s generated by nuclear power costs about twice as much as electricity generated through fossil fuel or hydroelectric plants. Another problem is that the supply of fissionable uranium-235 is limited. Of all the naturally occurring uranium, only about 0.75 percent is U-235. A vast majority is nonfissionable U-238. At current usage levels, we’ll be out of naturally occurring U-235 in fewer than 100 years. A little bit more time can be gained through the use of breeder reactors (see “Breeder reactors: Making more nuclear stuff,” later in this chapter). But there’s a limit to the amount of nuclear fuel available in the earth, just as there’s a limit to the amount of fossil fuels.
However, the two major problems associated with nuclear fission power are accidents (safety) and disposal of nuclear wastes.
Accidents: Three Mile Island and Chernobyl
Although nuclear power reactors really do have a good safety record, the distrust and fear associated with radiation make most people sensitive to safety issues and accidents. The most serious accident to occur in the United States happened in 1979 at the Three Mile Island Plant in Pennsylvania. A combination of operator error and equipment failure caused a loss of reactor core coolant. The loss of coolant led to a partial meltdown and the release of a small amount of radioactive gas. There was no loss of life or injury to plant personnel or the general population.
This was not the case at Chernobyl, Ukraine, in 1986. Human error, along with poor reactor design and engineering, contributed to a tremendous overheating of the reactor core, causing it to rupture. Two explosions and a fire resulted, blowing apart the core and scattering nuclear material into the atmosphere. A small amount of this material made its way to Europe and Asia. The area around the plant is still uninhabitable. The reactor has been encased in concrete, and it must remain that way for hundreds of years. Hundreds of people died. Many others felt the effect of radiation poisoning. Instances of thyroid cancer, possibly caused by the release of 1-13, have risen dramatically in the towns surrounding Chernobyl. It will be many more years until the effects of this disaster will be fully known.
How do you get rid of this stuff: Nuclear Wastes
The fission process produces large amounts of radioactive isotopes. If you look at Table 5-2, you’ll notice that some of the half-lives of radioactive isotopes are rather long. Those isotopes are safe after ten half-lives. The length of ten half-lives presents a problem when dealing with the waste products of a fission reactor.
Eventually, all reactors must have their nuclear fuel replenished. And as we disarm nuclear weapons, we must deal with their radioactive material. Many of these waste products have long half-lives. How do we safely store the isotopes until their residual radioactivity has dropped to safe limits (ten half-lives)? How do we protect the environment and ourselves, and our children for generations to come, from this waste? These questions are undoubtedly the most serious problem associated with the peaceful use of nuclear power.
Nuclear waste is divided into low-level and high-level material, based on the amount of radioactivity being emitted. In the United States, low-level wastes are stored at the site of generation or at special storage facilities. The wastes are basically buried and guarded at the sites. High-level wastes pose a much larger problem. They’re temporarily being stored at the site of generation, with plans to eventually seal the material in glass and then in drums. The material will then be stored underground in Nevada. At any rate, the waste must be kept safe and undisturbed for at least 10,000 years. Other countries face the same problems. There has been some dumping of nuclear material into deep trenches in the sea, but this practice has been discouraged by many nations.
Breeder reactors: Making more nuclear stuff
Only the U-235 isotope of uranium is fissionable because it’s the only isotope of uranium that produces the excess of neutrons needed to maintain a chain-reaction. The far more plentiful U-238 isotope doesn’t produce those extra neutrons.
The other commonly used fissionable isotope, plutonium-239 (Pu-239), is very rare in nature. But there’s a way to make Pu-239 from U-238 in a special fission reactor called a breeder reactor. Uranium-238 is first bombarded with a neutron to produce U-239, which decays to Pu-239. The process is shown in Figure 5-4.
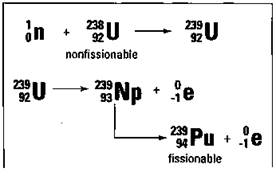
Figure 5-4: The breeder reactor process.
Breeder reactors can extend the supply of fissionable fuels for many, many years, and they’re currently being used in France. But the United States is moving slowly with the construction of breeder reactors because of several problems associated with them. First, they’re extremely expensive to build. Second, they produce large amounts of nuclear wastes. And, finally, the plutonium that’s produced is much more hazardous to handle than uranium and can easily be used in an atomic bomb.
Nuclear Fusion: The Hope for Our Energy Future
Soon after the fission process was discovered, another process, called fusion, was discovered. Fusion is essentially the opposite of fission. In fission, a heavy nucleus is split into smaller nuclei. With fusion, lighter nuclei are fused into a heavier nucleus.
The fusion process is the reaction that powers the sun. On the sun, in a series of nuclear reactions, four isotopes of hydrogen-1 are fused into a helium-4 with the release of a tremendous amount of energy. Here on earth, two other isotopes of hydrogen are used: H-2, called deuterium, and H-3, called tritium. Deuterium is a minor isotope of hydrogen, but it’s still relatively abundant. Tritium doesn’t occur naturally, but it can easily be produced by bombarding deuterium with a neutron. The fusion reaction is shown in the following equation:
![]()
The first demonstration of nuclear fusion — the hydrogen bomb — was conducted by the military. A hydrogen bomb is approximately 1,000 times as powerful as an ordinary atomic bomb.
The isotopes of hydrogen needed for the hydrogen bomb fusion reaction were placed around an ordinary fission bomb. The explosion of the fission bomb released the energy needed to provide the activation energy (the energy necessary to initiate, or start, the reaction) for the fusion process.
Control issues
The goal of scientists for the last 45 years has been the controlled release of energy from a fusion reaction. If the energy from a fusion reaction can be released slowly, it can be used to produce electricity. It will provide an unlimited supply of energy that has no wastes to deal with or contaminants to harm the atmosphere — simply non-polluting helium. But achieving this goal requires overcoming three problems:
ü Temperature
ü Time
ü Containment
Temperature
The fusion process requires an extremely high activation energy. Heat is used to provide the energy, but it takes a lot of heat to start the reaction. Scientists estimate that the sample of hydrogen isotopes must be heated to approximately 40,000,000 K. (K represents the Kelvin temperature scale. To get the Kelvin temperature, you add 273 to the Celsius temperature. Chapter 2 explains all about Kelvin and his pals Celsius and Fahrenheit.)
Now 40,000,000 K is hotter than the sun! At this temperature, the electrons have long since left the building; all that’s left is a positively-charged plasma, bare nuclei heated to a tremendously high temperature. Presently, scientists are trying to heat samples to this high temperature through two ways — magnetic fields and lasers. Neither one has yet achieved the necessary temperature.
Time
Time is the second problem scientists must overcome to achieve the controlled release of energy from fusion reactions. The charged nuclei must be held together close enough and long enough for the fusion reaction to start. Scientists estimate that the plasma needs to be held together at 40,000,000 K for about one second.
Containment
Containment is the major problem facing fusion research. At 40,000,000 K, everything is a gas. The best ceramics developed for the space program would vaporize when exposed to this temperature. Because the plasma has a charge, magnetic fields can be used to contain it — like a magnetic bottle. But if the bottle leaks, the reaction won’t take place. And scientists have yet to create a magnetic field that won’t allow the plasma to leak. Using lasers to zap the hydrogen isotope mixture and provide the necessary energy bypasses the containment problem. But scientists have not figured out how to protect the lasers themselves from the fusion reaction.
What the future holds
The latest estimates indicate that science is 5 to 10 years away from showing that fusion can work: This is the so-called break-even point; where we get out more energy than we put in. It will then be another 20 to 30 years before a functioning fusion reactor is developed. But scientists are optimistic that controlled fusion power will be achieved. The rewards are great — an unlimited source of nonpolluting energy.
An interesting by-product of fusion research is the fusion torch concept. With this idea, the fusion plasma, which must be cooled in order to produce steam, is used to incinerate garbage and solid wastes. Then the individual atoms and small molecules that are produced are collected and used as raw materials for industry. It seems like an ideal way to close the loop between waste and raw materials. Time will tell if this concept will eventually make it into practice.
Am I Glowing? The Effects of Radiation
Radiation can have two basic effects on the body:
ü It can destroy cells with heat.
ü It can ionize and fragment cells.
Radiation generates heat. This heat can destroy tissue, much like a sunburn does. In fact, the term radiation burn is commonly used to describe the destruction of skin and tissue due to heat.
The other major way that radiation can affect the body is through the ionization and fragmentation of cells. Radioactive particles and radiation have a lot of kinetic energy (energy of motion — see Chapter 2) associated with them. When these particles strike cells within the body, they can fragment (destroy) the cells or ionize the cells — turn the cells into ions (charged atoms) by knocking off an electron. (Flip to Chapter 3 for the full scoop on ions.) Ionization weakens bonds and can lead to the damage, destruction, or mutation of the cells.
Radon: Hiding in our houses
Radon is a radioactive isotope that's been receiving a lot of publicity recently. Radon-222 is formed naturally as part of the decay of uranium. It's an unreactive noble gas, so it escapes from the ground into the air. Because it’s heavier than air, it can accumulate in basements.
Radon itself has a short half-life of 3.8 days, but it decays to Polonium-218, a solid. So if radon is inhaled, solid Po-218 can accumulate in the lungs. Po-218 is an alpha emitter, and, even though this type of radiation is not very penetrating, it has been linked to increased instances of lung cancer. In many parts of the United States, radon testing is performed before selling a house. Commercial test kite can be opened, left in the basement area for a specified amount of time, and then sent to a lab for analysis. The question of whether radon represents a serious problem is stiff being investigated and debated.