The Handy Chemistry Answer Book (2014)
ATOMS AND MOLECULES
MOLECULES AND CHEMICAL BONDS
What is a molecule?
A molecule is a set of atoms held together by chemical bonds. Molecules are the smallest units of a substance that behave as that substance, and separating the atoms of a molecule will change its properties.
What is a substituent?
A substituent is an atom, or group of atoms, attached to a specific position in a molecule. For example, in the molecule 3-bromopentane (see drawing below), we could refer to the bromine as a substituent on the third carbon atom.
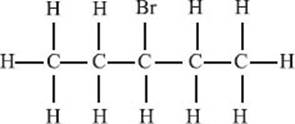
What is a chemical bond?
A chemical bond is an attractive interaction that binds atoms together through a sharing of electron density. The simplest bonding arrangement involves just two electrons shared between nuclei such that each effectively has a stable octet of eight valence electrons (or just two in the case of H–H). When two atoms are sharing a total of two electrons between them, the atoms are referred to as singly bonded to each other.
Bonds are what hold atoms together in molecules, and they are usually not easily broken. The arrangement of atoms in a molecule determines the identity of a chemical compound. The making or breaking of bonds is a chemical reaction, which converts one chemical compound into another.
Can I think of chemical bonds as springs between atoms?
Chemical bonds can be thought of as springs holding together the atoms in a bond. When atoms in a bond are stretched or compressed from their equilibrium separation, the bond provides a force to pull the atoms back together or to keep them from getting too close. For relatively small displacements, the bond actually provides a force that is physically very similar to that of a spring connecting two objects. This model of a spring as a chemical bond can be very useful for getting an intuitive idea of how a chemical bond connects atoms in a molecule.
What is a Lewis structure?
Lewis structures are a simple way of depicting the electronic structure of atoms and molecules. They show us which atoms are bonded to each other in a molecule and also show how many nonbonded electrons are present in the valence electron shell of each atom. The easiest way to understand them is probably to just take a look at a few.
The simplest Lewis structure is that for a single hydrogen atom. It has just one electron, and its Lewis structure looks like this:
![]()
The letter H lets us know that it’s a hydrogen atom, and the one dot represents its one electron.
Moving on to the Lewis structure for a molecule, let’s look at the Lewis structure for F2:
![]()
Here the two Fs let us know there are two fluorine atoms. The line connecting them shows that they are bonded with a single bond (containing two electrons). Each has six more electrons surrounding it, and these electrons are nonbonding.
And finally for a molecule with more than one bond, CH2O:
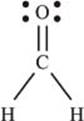
This molecule is called formaldehyde. The Lewis structure shows us that the carbon is involved in a single bond (sharing two electrons) with each hydrogen atom, and a double bond (sharing four electrons) with the oxygen atom. The oxygen atom also has four nonbonding electrons.
What is a “stable octet”?
The term “stable octet” describes the fact that many atoms in molecules are most stable when the valence shell contains effectively eight electrons. This counts both non-bonding electrons and electrons in chemical bonds between atoms. Molecules tend to be most stable when the valence shells of each atom in the molecule contain eight electrons. In the Lewis structures for F2 and CH2O (see the previous question), we see that the fluorine, carbon, and oxygen atoms are each surrounded by eight electrons. We get this total by adding both the nonbonding and bonding electrons. Since hydrogen atoms are in the first row and have just a single orbital in their valence shell, they only need two electrons (a single bond) to fulfill their analogue of a stable octet.
What is electronegativity?
Electronegativity is a property that describes the tendency of an atom to attract electrons in a chemical bond. The most electronegative atoms are those which “pull” hardest on the electron density they share in a bond with another atom. There is more than one scale and definition for electronegativity, and our description here follows that given by Linus Pauling, which is the most commonly used scale in chemistry courses. Electronegativity can most readily be described in terms of the number of protons in the nucleus of the atom and the distance to which its valence electron cloud extends away from the nucleus. As a general trend, the most electronegative atoms are those with the shortest distance between the valence electrons and the nucleus. Electronegativity isn’t a physical quantity that can be directly measured, but several scales have been developed that derive values for this property based on other measurable physical quantities.
What is polarity and how is it related to molecular structure?
Polarity is related to the symmetry of the arrangement of electron density in a molecule. Polar molecules are those which possess a net dipole moment, which means that the electron density is not symmetrically distributed in all directions. Nonpolar molecules have the electron density distributed in such a way that there is no net dipole moment. Typically this doesn’t mean that nonpolar molecules have their electron density distributed evenly over every part of the molecule, but rather that the dipole moments created by an unequal sharing of electrons in each individual bond cancel each other out, so that there is no net direction in which an asymmetry of electron density exists.
What is the charge of a molecule?
The overall charge of a molecule is determined by the number of protons and electrons in the whole molecule. If there are more protons than electrons, the molecule will possess an overall positive charge. If there are more electrons than protons, the molecule will similarly possess an overall negative charge. A molecule with the same number of electrons and protons is neutral and has no net charge.
How are formal charges different?
Formal charges are given for individual atoms within molecules. These are determined by dividing the electrons in every bond equally between the atoms that share them, regardless of the elements involved. Textbooks typically follow this somewhat obtuse statement with an equation (which always helps, right?) like this:
Formal Charge = Group Number – Nonbonding Electrons – ½ Bonding Electrons
Let’s work through this with an example, starting with carbon monoxide:
![]()
Carbon is in Group 4 of the periodic table; it has two nonbonding electrons (the two dots shown), and since there are three bonds to oxygen, there are six bonding electrons. So the formal charge is 4 – 2 – ½ (6), or –1. Oxygen is in Group 6 and has the same number of nonbonding and bonding electrons as carbon does in this example. The formal charge on oxygen is therefore 6 – 2 – ½ (6), or +1. Carbon monoxide has no net (or total) charge (because 1 + – 1 = 0), but the individual atoms do have formal charges.
What is Coulomb’s law?
Coulomb’s Law tells us the force experienced by a pair of separated charges. It’s a fundamental equation in the study of electrostatics, which is a broad area of physics concerned with the interactions between stationary charges. The equation for this force can be written:
![]()
where charges q1 and q2 are separated by a distance r12 and have a “unit of charge” defined by:
![]()
in which z is the charge in Coulomb’s and ε0 is the permittivity of free space, a fundamental physical constant.
The key features of Coulomb’s Law are that it predicts an attractive force between particles of opposite charge and that this force decreases with the square of the distance between the particles. For chemistry, it’s relevant to point out that the force between charges falls off rather slowly with the distance between them, so where charges are present in relatively dense materials (like liquids and solids), they have a significant effect on their environment.
What is a dielectric constant?
The dielectric constant of a material characterizes the extent to which it insulates against the flow of charge or against the effects of an electric field. Materials with a high dielectric constant screen the effects of charges within the material, while materials with a low dielectric constant allow the effects of a charge to be felt more strongly. In solutions containing ions, the dielectric constant of the solution will determine the extent to which the other molecules in the solution feel the effects of the charges present. The lowest possible dielectric constant exists in a vacuum in which there is no material present to screen the charge of a field.
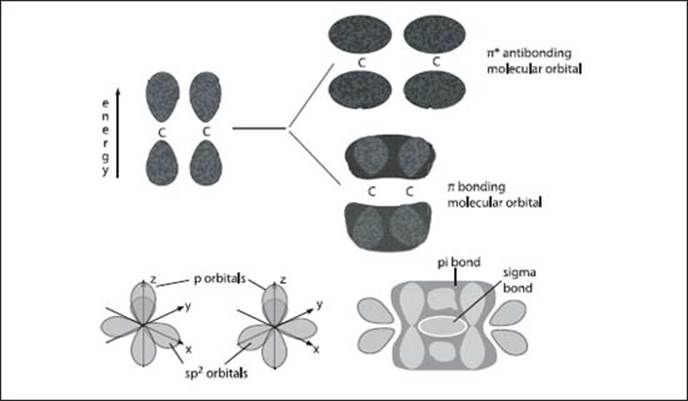
The top graphic (A) illustrates the pi-orbital formation from two p-orbitals; the bottom graphic (B) illustrates the formation of sigma- and pi-molecular orbitals from two sp2 hybridized carbon atoms.
What is valence bond theory?
Valence bond theory is one of two main theories (the other being molecular orbital theory) that is used to explain bonding in molecules. Valence bond theory explains bonding by describing the interactions of atomic orbitals on individual atoms as they come together to form chemical bonds. The basic idea is that orbitals with the right shapes to overlap strongly with each other will form the strongest chemical bonds. Today, valence bond theory’s description of chemical bonding based on atomic orbitals has become less popular in favor of molecular orbital theory.
What are molecular orbitals?
Molecular orbitals are different from atomic orbitals in that they cover several atoms and possibly even a whole molecule. While atomic orbitals originate from a single atom, molecular orbitals are formed from combinations of the atomic orbitals. Because they allow electrons to occupy the space between the atoms in a molecule, they can provide a very useful description of chemical bonds holding atoms together.
What is molecular orbital theory?
Molecular orbital theory is the other main theory (the first was valence bond theory) used to explain and predict bonding properties in molecules. Molecular orbital theory describes bonding interactions by using molecular orbitals that are spread out over multiple atoms, and this allows an electron’s location to be described by an orbital that bonds atoms together in a more realistic way than valence bond theory.
What are some common structures/geometries for molecules?
The study of chemistry has benefited greatly from knowledge of properties relating to the geometries and, especially, the symmetries of molecules. To get a sense of what shapes molecules adopt, it’s worth taking a look at a few of the geometries that come up often in the study of chemistry.
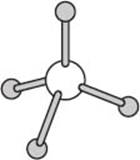
One commonly encountered geometry is that of a tetrahedron. Methane has the molecular formula CH4 and exists in a tetrahedral geometry with angles of approximately 109 degrees between each pair of C–H bonds.
![]()
Linear geometries are also relatively common. Carbon dioxide has the molecular formula CO2 and exists in a linear geometry with a 180-degree angle between the CO bonds.
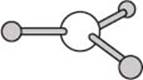
One last geometry we’ll look at here is a planar geometry. The molecule BH3 provides one example of a planar geometry, and in this case the BH bonds are separated by angles of 120 degrees. There are also planar molecules with four bonds in a plane, and in those cases the bonds are separated by angles of 90 degrees.
How large are molecules?
Molecules span a wide range of sizes. The smallest molecules contain only two atoms, and these diatomic molecules have length scales that are approximately the sum of the atomic radii of the constituent atoms. The smallest molecule, H–H, has a length of only 0.74 Ångströms (7.4 × 10−11 m). Larger molecules can be comparatively quite large. Biologically important molecules, like proteins, often contain thousands of atoms. Polymers, which are highly linked networks of covalently bonded atoms, can be even larger still, sometimes becoming so large they are visible to the naked eye.
Is it possible to see a single molecule?
With some of the largest single molecules, like polymers, they can actually be seen by the naked eye or through a microscope. Most molecules, however, are so small that a single isolated molecule cannot be seen with even the best microscopes. There is a physical limitation that prevents their observation with light, which has to do with the size of small molecules (lengths of ca. 0.1 to 1.0 nm) being significantly smaller than the wavelengths of visible light (400 to 700 nm). Other techniques based on diffracting electrons off of molecules, measuring the force molecules exert against a very small metal tip, and other methods have been developed to image small molecules, but it’s impossible to see most small molecules with light in the way that we conventionally see things.
Is everything made of molecules or atoms?
Basically, yes! The only material things that aren’t made up of atoms or molecules are the subatomic particles that make up atoms. Anything you find around your house, office, or anywhere else is made of some combination of atoms that are on the periodic table.
How do molecules interact?
The forces molecules exert on each other fall into a few main categories:
Van der Waal’s interactions—Van der Waal’s interactions are the broadest group of intermolecular interactions. This includes basically all attractive and repulsive forces that don’t involve ions (charged atoms or molecules) or the rather unique situation of hydrogen bonding. Van der Waal’s interactions include forces due to the dipole moments of polar molecules as well as interactions due to induced dipoles that can form even in nonpolar molecules.
Ionic interactions—Another class of intermolecular attractions involves attractive and repulsive forces between pairs of ions, or between ions and neutrally charged atoms or molecules. These interactions are typically stronger than those in the Van der Waal’s category. Interactions between pairs of ions are governed by Coulomb’s law, while interactions between ions and neutral molecules are either ion-dipole or ion-induced dipole interactions.
Hydrogen bonding—A hydrogen bond is a strong interaction between a hydrogen atom and another electronegative atom (usually fluorine, oxygen, or nitrogen) that are not covalently bonded to one another. The hydrogen atom also must typically be bonded to an electronegative atom (usually oxygen or nitrogen). The origin of this strong attractive interaction is that a hydrogen atom bonded to an electronegative atom has a partial positive charge due to its lack of electron density. This allows the hydrogen atom to have a strong attractive interaction with electronegative atoms (or ions), which have partial negative charges due to their extra electron density.
How strong are intermolecular interactions relative to a covalent bond?
Most intermolecular interactions are fairly weak relative to a covalent bond. Covalent bonds typically involve energies on the order of 100 kilocalories per mole (a unit of energy commonly used in chemistry). Van der Waal’s interactions are the weakest type of intermolecular interaction with typical energies of roughly 0.01 to 1 kilocalories per mole (or 0.01% to 1% the strength of a covalent chemical bond) for a pair of interacting atoms. The strengths of ion-ion and ion-dipole interactions can vary widely, particularly in solutions, because the ions and/or dipoles can be separated by very different distances. The charges of ions can also be significantly shielded by solvent molecules around them. If the ions are very close together (like in a solid), their interaction energy can approach (or even exceed) that of a covalent bond. Hydrogen bonds are usually the strongest type of intermolecular interaction with energies of about 2–5 kilocalories per mole (or roughly 2% to 5% the strength of a covalent bond). Because they are such strong interactions, hydrogen bonds can play a dominant role in determining the structures of liquids, solids, and single molecules.
What’s a solvent?
In chemistry, a solvent is a liquid (though it can be a gas or a solid, but forget about that for now) that other chemicals are dissolved in. These other chemicals can be called the solute. The solute and the solvent can together be called a solution. Take salt water: water is the solvent, salt is the solute, and we can refer to the salt water as a solution.
What makes something magnetic?
As we mentioned just briefly above, electrons have a property called a spin, or spin angular momentum, which can take on two possible values. This property, combined with the fact that electrons are charged particles, dictates that each electron has an associated magnetic moment, called the spin magnetic moment. In a macroscopic object, magnetism, or lack thereof, is determined by whether these spin magnetic moments are all aligned in the same direction. If all of the spin magnetic moments line up in the same orientation, the object will behave as a magnet. If the spin magnetic moments are oriented randomly, the object won’t be magnetic. The trick is that only certain materials have the potential to exhibit magnetism, and we’ll get to that next.
What determines which metals can be magnetized?
Chemists discuss magnetism in terms of three basic categories: diamagnetism, paramagnetism, and ferromagnetism.
Diamagnetic materials have all of their electrons arranged in pairs, which, by definition, means that their spin magnetic moments must all be arranged in pairs and thus cancel each other out. For this reason, diamagnetic materials cannot be magnetic, and won’t be influenced by magnetic fields.
Paramagnetic materials have unpaired electrons, but in these materials the electrons’ spin magnetic moments cannot all be lined up in the same direction, which means that they cannot be strongly magnetic. Since they do have unpaired electrons, they can be influenced by applied magnetic fields, but not to the same extent as the third class, ferromagnetic materials.
Ferromagnetic materials are the materials that can give rise to the magnets we’re all familiar with. All of the materials that magnets can interact strongly with are ferromagnetic. In these materials, there are unpaired electrons whose magnetic spin can all be aligned in the same direction. Note that just because a material is ferromagnetic doesn’t mean it must be a magnet, but rather just that it has the potential to be magnetized. Take a paper clip, for example; when you first pick it up it’s not a magnet, but you can turn it into a weak magnet by holding a magnet next to it for a short length of time. Some of the most common ferromagnetic substances are those made of iron, nickel, or cobalt.
What is an ideal gas?
An ideal gas is a collection of atoms or molecules that do not interact with one another and occupy essentially no volume. While this is an idealized model, it turns out to describe many gases very well. The reason it works so well is that the atoms or molecules making up a gas are spread out far from one another so that the intermolecular forces between them are extremely weak (they don’t “feel” each other). This description leads to the ideal gas law, which is a relationship between the pressure, volume, and temperature of a gas. The ideal gas law allows chemists to predict how, for example, the volume of a gas will change as its temperature is increased. The equation for the ideal gas law is
PV = NkbT
where P is pressure, V is volume, N is the number of particles (atoms or molecules), T is temperature, and kb is Boltzmann’s constant (a fundamental physical constant).
How many different chemical substances have been discovered?
According to the Chemical Abstracts Service (CAS), which is the world’s largest authority for chemical information, the seventy millionth chemical compound was recently registered (announced December 2012). Today, new chemicals are being discovered and registered at a staggering rate: the sixty millionth chemical was registered only eighteen months earlier!