Industrial Chemistry: For Advanced Students - Mark A. Benvenuto 2015
Lithium
The least dense metal on the periodic table, lithium, has a density of 0.534 g/cm3, which is even less than that of aluminum (2.699 g/cm3), another light metal produced on an industrial scale. This property alone makes lithium an extremely useful metal in numerous applications where minimizing total weight is either necessary or desired. Since it can only gain or lose one electron, it is also useful in applications and systems where no over-oxidation can be tolerated.
14.1 Mining and sources
There is one brine operation in the United States, in Nevada, from which lithium is extracted, at Searles Lake. But the figures from this operation are not reported to the United States Geological Survey, to protect proprietary, corporate data (USGS, 2014). The entire world’s production, less this Nevada operation, is shown in Figure 14.1 in terms of metric tons. This pie chart does not mention two nations, Bolivia and Afghanistan, which have or are estimated to have enormous reserves of lithium minerals.
In the case of Bolivia, large-scale mining and production of lithium in the Uyuni salt flats area has only just begun. There is no infrastructure in Bolivia at present that can rapidly move to extraction of lithium salts and minerals on a large scale; and thus the Bolivian government is negotiating with other firms and foreign governments for rights to extract and export lithium materials.
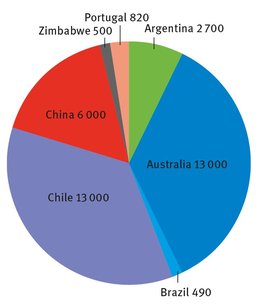
Fig.14.1: Worldwide lithium sources USGS (2014).
In the case of Afghanistan, an official Soviet document outlining possible reserves of numerous minerals including lithium apparently resurfaced after the United States military intervention in the country (Minerals In Afghanistan, 2014). But no mining company or concern has yet begun any extraction of spodumene or any other lithium-bearing minerals or salts.
14.2 Extraction chemistry
There are several lithium ores, as well as large deposits of what is best called lithium brine in certain parts of the world. Spodumene, a lithium aluminum silicate, is one ore that is refined into useable lithium salts. The chemistry can be represented as shown in Figure 14.2.

Fig. 14.2: Lithium extraction from ore.
Lithium chloride can then be mixed with potassium chloride in a 55:45 ratio and elec-trolysized at 450°C to reduce the lithium.
![]()
This process seems cumbersome at first, but since there are few lithium salts that occur as simple mineral structures, it is the necessary purification route. Also, production and isolation of lithium carbonate is important when producing many types of lithium-based batteries.
There are estimates and predictions that in the future the isolation of lithium chloride from brines in the countries listed in Figure 14.1 will make the production of lithium salts and lithium metal economically more attractive, but these remain speculative at the present. Figure 14.3 shows the simplified reaction chemistry that produces lithium hydroxide, a valuable commodity.
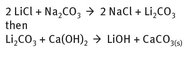
Fig. 14.3: Lithium hydroxide production.
Table 14.1: Uses of lithium (USGS, 2014).
Area of use |
Amount (%) |
Comments |
Ceramics and glass |
30 |
Li2CO3 is used in firing, becoming Li2O (Digitalfire Ceramic Materials Database, 2014) |
Batteries |
22 |
Small, rechargeable, for portable electronics, as well as electric vehicle batteries (Western Lithium, 2014; International Lithium Corporation, 2014) |
Lubricating greases |
11 |
Lithium 12-hydroxystearate, high melting point |
Air treatment |
4 |
“Lithium bromide brine (54%) is used as a stable, chlorofluoro-carbon-free absorption medium.” (Rockwood Lithium, 2014) |
Metallurgical |
4 |
Flux |
Polymers |
3 |
Alkyl lithium, catalyst or initiator. |
Pharmaceuticals |
2 |
Carbon—carbon bond formation |
Primary aluminum production |
1 |
|
Military fuels |
<1 |
LiAlH4 , rocket propellant additive |
Other |
23 |
14.3 Uses
There has been a fair amount of coverage in the general media in the past 5 years about the use of lithium as a battery material, especially about its potential for use as a new generation of batteries for electric automobiles. There are even now Electric Vehicle Associations and networks (Electric vehicle organizations, 2014). But the profile for uses of lithium and lithium salts is somewhat greater than simply this one use. It can be seen in Table 14.1.
14.3.1 Ceramics and glass
As mentioned in Table 14.1, it is lithium carbonate that can be utilized in the production of various ceramics and glasses. The firing process effects the transformation to lithium oxide. However, lithium oxide itself is used as a flux in glass making, since it results in glasses with small coefficients of thermal expansion. This makes the resulting glasses useful in consumer end-use materials such as ovenware, in which the object must be able to withstand repeated heating and cooling cycles.
14.3.2 Lithium batteries
Lithium battery chemistry does not involve reduced lithium metal, but rather utilizes lithium—cobalt salts. Lithium—cobalt oxide is the current mixed metal salt used with graphite in many lithium batteries. The reaction chemistry for the charge and discharge of one type of lithium battery can be shown as seen in Figure 14.4.
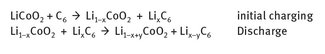
Fig. 14.4: Lithium transfer in lithium—cobalt oxide batteries.
The first reaction illustrates the loss of some lithium into the graphite. Since the amount lost is not precise, it is simply designated as “x.” The second reaction shows the discharge, and the return of a somewhat smaller amount of lithium back to the lithium—cobalt salt. Once again, this amount is not known precisely, and thus is designated as “y.”
The LiCoO2 itself must be synthesized at 900 °C, and contains Li+ and Co3+ between planes of O atoms. Lithium—air batteries represent somewhat different reaction chemistry than that shown above, in part because of the different environments in which they can run. For example, in an acidic electrolytic environment, lithium oxidation occurs as shown in Figure 14.5.
![]()
Fig. 14.5: Lithium—air battery, acidic reaction.
The subsequent reduction of the oxidized lithium sometimes produces lithium hydroxide. Lithium—air batteries can also function in a basic or alkaline environment, as shown in Figure 14.6.
![]()
Fig. 14.6: Lithium—air battery, basic reaction.
As shown, water must participate in the reaction when the metal oxidation occurs. In both of the above cases, different cathode materials are being developed to effect the reduction of lithium from its oxidized state (Lithium Air Industries, 2014).
14.3.3 Lubricating greases
There is certainly no overarching theory concerning the production of greases and lubricants. Rather, any specific grease is used simply because it works well in a known, defined application. For example, lithium 12-hydroxystearate is produced on a large scale because it has desirable viscosity, melting point, and other characteristics for several applications. Figure 14.7 shows the Lewis structure of the molecule.
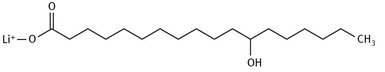
Fig. 14.7: Structure of lithium 12-hydroxystearate.
This stearate is made by adding lithium hydroxide slowly to a heated solution of the hydroxyl-stearic acid. In turn, this fatty acid can be extracted from the triglycerides in castor oil.
14.3.4 Air treatment
Lithium compounds are at times used in heating, ventilation, and air-conditioning systems (HVAC). The reason lithium salts are used in large air treatment operations (the HVAC systems of large commercial buildings, for example) is because of the dessi-cant capabilities of several lithium salts. Given sufficient time, lithium salts, such as lithium chloride, can actually pull enough moisture from air that they solvate entirely, resulting in a brine solution, a laboratory-scale example of which is shown in Figure 14.8. This broken container of one pound of lithium chloride was simply stored in the beaker, as shown, for slightly more than 1 year. It was originally a white powder and eventually became a brine solution. The lithium chloride brine level is noted, as is a break in the jar.
Perhaps obviously, in commercial building HVAC systems, lithium salts scavenge moisture, but must then be able to release it to an exhaust vent, in the process redrying the lithium salt.

Fig. 14.8: Broken container of lithium chloride after 1 year.
14.4 Potential uses
The lithium battery technology outlined above, as well as several other lithium-containing materials, has already been proved to be effective as batteries in small electronic devices and in much larger batteries used in battery-powered vehicles (automobiles and light trucks). However, whether such processes will be scaled up to meet the demands of the developed world for automotive transport remains in question. Current internal combustion engines and gas tanks generally enable a trip of 300 miles without refueling, acceleration from 0—60 miles per hour (0—100 kph) in less than 15 s, and refueling stops that last less than 10 min. These specifications will have to be met for any electric vehicle to be commercially successful, simply because consumers are now accustomed to these standards and do not wish to compromise them. Currently, electric vehicles do not have this range, recharging times are significantly longer than 10 min, and there is no infrastructure for “recharging stations” throughout the country as there is for gas stations. Should these technical hurdles be overcome though, the potential for electric vehicles to claim a large share of the automotive market is great, and has the potential to change the world’s economy.
14.5 Recycling
Since lithium has such a broad profile of applications and uses, and since some of it includes very small consumer end-use devices, it is difficult to think that all of it can be recycled. Lithium batteries however can definitely be recycled. At the moment, small lithium batteries in portable electronics are not necessarily recyclable, simply because the cost of doing so is too high based on the size of the unit — meaning each battery is very small. But the companies that are marketing automobiles powered by lithium batteries have plans for large-scale recycling operations as the cars and the batteries reach the end of their useful life, or have plans for a second use of the batteries asas connected, stationary, and large power storage units.
As the recycling of large lithium batteries becomes established, a great deal of political interest in such processes may develop, since criticism has already been voiced that a total shift from gasoline-powered automobiles to battery powered automobiles may simply equate to a shift from dependence on Middle-East oil to a dependence on South American lithium. What makes this unpalatable to many users and political policy makers is that the governments in both areas do not necessarily have agendas and human rights records that are in harmony with the countries in which the end products — gasoline or lithium batteries — are largely being used.
Bibliography
Digitalfire Ceramic Materials Database. Website. (Accessed 17 January 2014, as: http://digitalfire.com/4sight/material/lithium_carbonate_975.html).
Electric vehicle organizations. a. Electric Drive Transportation Association. Website. (Accessed 20 May 2014, as: http://www.electricdrive.org/). b. Electric Auto Association. Website. (Accessed 20 May 2014, as: http://www.electricauto.org/). c. Plug In America. Website. (Accessed 20 May 2014, as: http://www.pluginamerica.org/). d. Light Electric Vehicle Association. Website. (Accessed 20 May 2014, as: http://www.levassociation.com/).
International Lithium Corporation. Website. (Accessed 20 May, 2014, as: http://www.internationallithium.com).
Lithium Air Industries. Website. (Accessed 20 May 2014, as: http://www.lithiumair.us/).
Minerals In Afghanistan. Website. (Accessed 19 May 2014, as: http://www.bgs.ac.uk/AfghanMinerals/docs/RareMetals_A4.pdf).
Rockwood Lithium. Website. (Accessed 18 January 2014, as: http://www.rockwoodlithium.com/applications/air-conditioners-gas-and-air-treatment/).
USGS Mineral Commodities Summary 2013. Website. (Accessed 20 May, 2014, as: http://minerals.usgs.gov/minerals/pubs/mcs/2013/mcs2013.pdf).
Western Lithium. Website. (Accessed 17 January 2014, as: http://www.westernlithium.com/what-is-lithium/what-is-it-used-for).