Industrial Chemistry: For Advanced Students - Mark A. Benvenuto 2015
Sodium
Over the past 50 years, reduced sodium metal has become a large commodity material and an economically useful one. Prior to this, while small amounts of the metal were produced, sodium existed and was being used in its oxidized form, usually as common table salt, although salts such as sodium hydroxide were known and utilized. Common salt has an ancient history and myriad uses, but here we will only discuss the production and use of sodium metal.
16.1 Sources
Sodium occurs in the Earth and oceans as sodium chloride. While there are other sodium-containing salts and minerals, sodium chloride is by far the the most abundant, and is either mined or evaporated from briny water, and then either used as is or reacted chemically to produce other materials, one of them being the sodium metal.
Salt is so common throughout the world that while the USGS Mineral Commodity Summaries tracks it each year and a Salt Institute is devoted to promoting its many uses (USGS, 2014; Salt Institute, 2014), as well as those of soda ash and sodium carbonate, there appears to be no fear that sodium chloride will ever be depleted.
16.2 Extraction chemistry
The electrolysis of salt water does not produce sodium metal. Rather, it produces sodium hydroxide, hydrogen gas, and chlorine gas. The reaction chemistry is fairly simple, and is
![]()
This is indeed a major industrial process, the chlor-alkali process, in which all three products are used.
The current industrial process by which sodium metal is isolated is an electrolysis of molten sodium chloride. This is a smaller process globally than many of the others we have examined, but still produces approximately 100,000 tons of the metal each year. The process occurs in a refractory chamber, called a Downs cell. The scheme is shown in Figure 16.1. The reaction chemistry for the Downs cell is deceptively simple, as shown:
![]()
What the reaction does not show is the energy input needed to make this reaction occur. The cell is normally kept at approximately 600 °C, and even at that temperature the starting material must be a mixture of sodium chloride and calcium chloride.
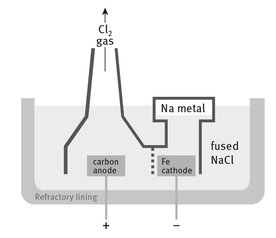
Fig. 16.1: Downs cell.
Without the latter, the temperature would have to be even higher. However, calcium chloride is so different in electronegativity that only sodium is reduced from the mixture. Both products are less dense than the molten mixture, and thus rise to the top, as shown in Figure 16.1.
The Castner process is older and somewhat more expensive than the use of Downs cells for sodium production, and thus has been replaced. But it represents a different way to produce sodium metal, starting with sodium hydroxide. It too is an electrolysis. The reaction chemistry runs at 330 °C and can be represented as
![]()
While the production of water, listed on the same side of the reaction as the sodium product, seems impossible, the water is dissolved in the molten electrolyte and thus does not ignite the reduced metal. However, build-up of small amounts of water does decrease the yield of the product.
The Deville process has also been entirely replaced by the use of Downs cells, but it does represent the earliest large-scale isolation of sodium. Sodium carbonate is the starting material instead of sodium chloride, and the reaction chemistry can be expressed as follows:
![]()
In the reaction, carbon is used as the reducing agent, and the entire reaction takes place at approximately 1100°C.
16.3 Uses
Uses of sodium metal, or for it as a starting material for further chemicals, are listed in Table 16.1. Major uses are listed here, as the profile becomes rather wide when all the small or niche uses are considered.
Table 16.1: Uses for sodium.
Material |
Use |
Comments |
Sodium metal |
Heat transfer fluid |
Used in nuclear reactors, has high thermal conductivity |
Sodium metal |
Vapor lamps |
Used in street lighting |
Sodium fusion test |
An older qualitative test for nitrogen, sulfur, and halogens in organic compounds. |
|
Alloys |
NaK alloys are used as oxygen scavengers |
|
Sodium amide |
Indigo dye |
Used in Pfleger’s synthetic method |
|
Sodium azide |
Automotive airbags |
Wislicenus process: |
2NH3 + 2Na → 2NaNH2 + H2 Then |
||
2NaNH2 + N2O → NaN3 + NaOH + NH3 |
||
|
Sodium borohydride |
Pulp industry, dyeing industry |
Made via: |
4NaH + B(OCH3)3 → 3NaOCH3 + NaBH4 |
||
|
Sodium hydride |
Production of sodium borohydride |
Formed by direct addition: |
2Na + H2 → 2NaH |
||
Tri-phenyl phosphine |
Organic syntheses |
6Na + PCl3 + 3PhCl → PPh3 + 6NaCl |
16.4 Other, heavy alkali metal production and uses
Of the other alkali metals, only potassium is present and reachable in the Earth’s crust in large enough quantities to be mined and extracted. Potash (potassium hydroxide, KOH) is by far the most common potassium-containing mineral, although there are several more. When pure potassium is needed, it can be separated from potassium chloride by what is called the thermal method, using sodium, as shown here:
![]()
It can also be separated from its fluoride by what is called the Griesheimer process, which utilizes calcium carbide, as shown here:
![]()
Industrially, potassium is used almost exclusively as one of the potassium salts. These account for its use in fertilizer, gunpowder, and numerous other applications. The production of potassium metal is a much smaller scale operation than the production of sodium metal.
16.5 Recycling
As with many of the materials discussed in this book, sodium is so often used in producing other materials that there is no recycling of any of it.it.
Bibliography
Salt Institute. Website. (Accessed 20 May, 2014, as: http://www.saltinstitute.org).
USGS Mineral Commodities Summary 2013. Website. (Accessed 20 May, 2014, as: http://minerals.usgs.gov/minerals/pubs/mcs/2013/mcs2013.pdf).