Industrial Chemistry: For Advanced Students - Mark A. Benvenuto 2015
Rare earth elements
We will use the older term “rare earth” here to describe and encompass all the lanthanide elements as well as yttrium and scandium. This general definition is that used by the International Union of Pure and Applied Chemistry (IUPAC), with the latter two elements included with the lanthanides because of their co-occurrence in many lanthanide-bearing ores. These elements are also called the lanthanides, the lanthanoids, and the inner transition metals, depending upon the publication that is discussing them.
Despite this older name, the rare earth elements are not particularly rare; rather, they are not concentrated in small areas where a mining operation is able to extract just one from a specific location. Their relative abundance in the Earth’s crust, in relation to several other elements, is shown in Table 18.1. It can be seen that some elements that find industrial scale uses are actually believed to be present in the Earth’s crust in lower abundances than many of the rare earth elements. Examples include bromine and uranium, shown at the bottom of Table 18.1. Even tin is less common than several of the rare earth elements.
Table 18.1: Abundance of the rare earth elements.
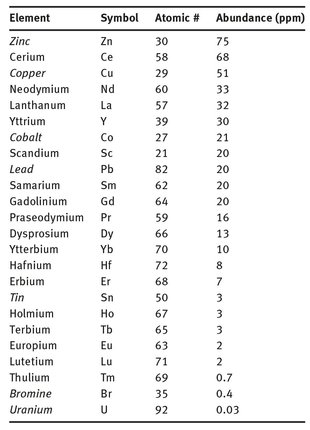
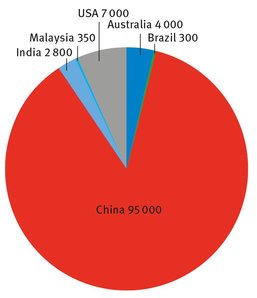
Fig. 18.1: Rare earth element producers (in metric tons of rare earth oxides).
18.1 Sources
While the United States has areas in the Western states from which rare earth elements can be extracted profitably, these have not been utilized in recent years. Rather, rare earth elements have been imported, mostly from China, in large part because such imports are less expensive. The worldwide producers of rare earth metals are shown in Figure 18.1 (US Geological Survey, 2013; RARE, 2014).
18.2 Extraction chemistry
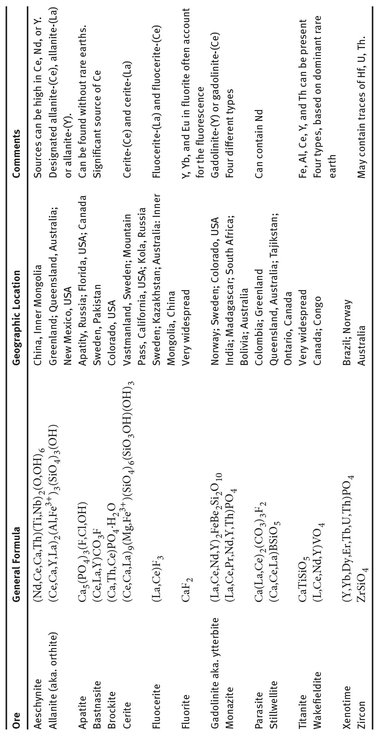
The extraction chemistry for the rare earth elements remains a challenge, because the reactivity of each element within this set is very close to that of the others, especially those immediately adjacent to it (Holleman and Wiberg, 1995). This is obvious when one looks at the ores from which these elements are refined. The most common ores are generally monazite, bastnasite, and xenotime, but there are several others. Table 18.2 shows the ores containing rare earth elements, and what are their general compositions. The complexity of several of the ores is obvious by their formulae. This also contributes to the difficulty of extracting specific elements from the various ores. But even simple ores, such as fluorite, can contain significant amounts of one rare earth element or another.
18.2.1 Separation and isolation
As mentioned, the separation of individual lanthanides from complex ore mixtures is still a challenge. The ionic radii of these elements are all very close to each other, but methods have been developed for their extraction and isolation. It is not possible to show simple reaction chemistry that illustrates the separation and isolation of each element because there are few cases when all of the lanthanides are present in a single ore sample. Rather, these complex separations are best explained in terms of a list of procedures, as follows:
Table 18.2: Ores containing rare earth elements.
1. Milling. As with many separation processes for various ores, the first step in this form of extraction is to bring all the material down to a uniform size. This is a mechanical process that crushes larger pieces of any rare earth-containing ore to smaller sizes, with a large surface area. Through crushing and sieving, the ore can be reduced in particle size to that of rough sands.
2. Division by electromagnetic means. A conveyor belt system is used to separate the magnetic substances in any ore from the nonmagnetic ones. This is accomplished by having electromagnets attached to one of the end rollers about which the belt spins. As material drops off the end of the belt to receiver bins, that which is non-magnetic simply drops, while the magnetic material is attracted to the magnets on the rotary wheel at the end of the belt. This material only drops away when the belt pulls it far enough away from the magnets, as seen in Figure 18.2.
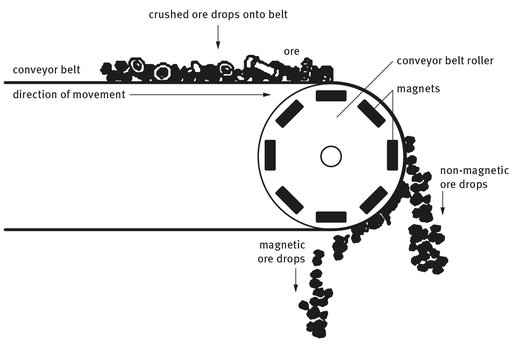
Fig. 18.2: Magnetic ore separation from non-magnetic ore.
3. Flotation. As with the separation of many ores, flotation is a further separation technique based on the density of the batch of ore. The ore is reduced to powder and put into an aqueous solution, usually one that has some surfactant material in the solution as well. Air is blown into the solution, which can separate the finer particles that do not sink.
4. Centrifugal concentration. By spinning the remaining material at high speedsinin especially designed centrifuges, the ore can be further separated by the density of the different materials within the batch.
5. Leaching and precipitation. The concentrated material can now be separated either as oxides or in some cases as metals, using the following techniques:
o (a) Fractional crystallization. This technique depends on the solubility differences of the various rare earth element salts in the solution. By adjusting temperature and pH carefully, selective precipitation occurs.
o (b) Ion exchange. As the name implies, this technique binds rare earth element ions to some medium, often a synthetic plastic resin or a synthetic zeolite, and solvates other ions into the solution. The rare earth-bearing materials are then washed and treated with acid to isolate the desired products from any other ionic materials remaining in the solution.
o (c) Extraction via solvents. The lanthanides, as soluble salts such as nitrates, are extracted from an aqueous solution into an organic one, such as kerosene, which has a chelating agent in it. One such chelating agent that has seen extensive use is tri-n-butylphosphate. This forms stronger complexes with the rare earth elements that have smaller ionic radii, making those more soluble in the organic phase.
6. Electrolysis. Electrolytic deposition of metals can occur if the metal salts are soluble, and if an anodic material can be made soluble during the reduction of the desired rare earth element.
It should be emphasized that each of these steps may sometimes need to be performed numerous times to achieve complete separation of one of the rare earth elements from another. In some cases, depending upon the batch of ore, a step may need to be performed hundreds of times to concentrate one element and deplete another from a particular solution or precipitate.
18.2.2 Refining to pure rare earth elements
While all of the rare earth elements can be reduced to their metal states, many applications of them do not require this. Table 18.3 gives a brief list of most of the major uses of rare earth elements. It becomes apparent that many of them are used as oxides or some other compound.
Table 18.3: Uses of the rare earth elements.
Name |
Use |
Form |
Scandium |
Aerospace metal alloys |
Sc−Al alloy |
Yttrium |
Ceramics, phosphors, alloys |
EuYVO4 for phosphors |
|
Lanthanum |
Batteries, catalysts for petroleum refining |
NiMH battery component |
Glass |
AsLaF3 |
|
|
Cerium |
Catalysts, auto |
Ce2O3 |
Oven cleaning catalyst |
Ce2O3 |
|
Optical polishing |
CeO2 |
|
Ceramics |
CeO2 |
|
Optician’s rouge |
CeO2 |
|
Gas mantles |
CeO2 |
|
Alloys |
Ce metal |
|
|
Praseodymium |
Magnet alloys |
Pr−Nd metal |
Carbon arc lights |
(La)Fx |
|
Glass |
Mixed |
|
Fiber optic amplifier |
Mixed |
|
Mischmetal |
Pr, Ce, La, Nd |
|
Neodymium |
Magnets |
NdFeB alloy |
Promethium |
Beta radiation emitter |
As oxide or chloride |
|
Samarium |
Magnets |
Sm−Co alloy |
Reactor control rods |
Alloy component |
|
Europium |
Liquid crystal displays |
Eu2O3 |
Gadolinium |
Numerous niche uses |
Usually as alloy component, or as oxide |
|
Terbium |
Phosphors |
Tb:Gd2O2S |
Magnets |
Minor component |
|
|
Dysprosium |
Magnets |
Nd−Fe−B−Dy, up to 5% Dy |
Lasers |
Dy−V |
|
Holmium |
High power magnets |
As alloy |
|
Erbium |
Lasers |
Y3Al5O12: Er |
Specialty glasses |
As a dopant |
|
|
Thulium |
X-ray device source |
As reduced metal |
Lasers |
Tm:Ho:Cr:Y3Al5O12 |
|
|
Ytterbium |
Stainless steel |
Minor dopant |
Fiber optics |
As dopant |
|
Lutetium |
Few commercial uses |
18.3 Uses
The profile of uses for the rare earth elements is almost as wide and varied as the composition of the ores from which they are refined. Perhaps the most obvious recent use of any of the rare earth elements is as a component of the small magnets that are incorporated into cellular phones. Without the use of such magnets, cell phones would conceivably still be available in the size of the portable phones first commercialized in the 1980s. Table 18.3 shows common uses for each lanthanide. Many are niche uses.
It can be seen from Table 18.3 that several of these elements find use in high strength magnets, as well as in various types of lasers. Indeed, such products continue to be used in more niche applications each year, prompting warnings from the United States Department of Energy Ames Research Lab about the perceived loss of technical ability in this area within the United States. Leading expert Dr Karl Gschneider is particularly keen on ensuring that future scientists are trained in the use and refining of rare earth elements, and able to make advances in such fields (US Department of Energy, 2014).
18.4 Recycling
The recycling of rare earth elements is still in its infancy, although some commercial efforts have been made in Japan (Japan Recycles Minerals, 2010). One of the difficulties in large-scale recycling of rare earth elements is simply removing them from the user end products in which they reside in some economically feasible manner. This is because the amount used per unit is usually quite small, such as the magnet material in a cell phone. Some efforts at recycling items such as cell phones are already underway, though. Figure 18.3 shows one such recycling kiosk in a shopping mall near Detroit, Michigan, USA.

Fig. 18.3: Automated cell phone recycling kiosk.
As with most recycling programs, the incentive is economic rather than one of scarcity of the materials. In general, it is believed that there is more than a century of rare earth elements left that can be extracted from ores. But recycling may prove to be less expensive.
Bibliography
Holleman, Wiberg. Inorganic Chemistry, 1995, DeGruyter.
Japan Recycles Minerals from Used Electronics, New York Times, 2010. Website. (Accessed 15 January, 2014, as: http://www.nytimes.com/2010/10/05/business/global/05recycle.html?pagewanted=all&_r=0).
RARE, The Association for Rare Earths. Website. (Accessed 5 June 2014 as: http://www.rareearthassociation.org).
US Department of Energy, Ames Research Laboratory. Website. (Accessed 5 June 2014, as: https://www.ameslab.gov/news/inquiry/2010-2-mr-rareearth).
US Geological Survey, Mineral Commodity Summaries 2013. Website. (Accessed 26 January 2014, as: http://minerals.usgs.gov/minerals/pubs/mcs/2013/mcs2013.pdf).