Industrial Chemistry: For Advanced Students - Mark A. Benvenuto 2015
Thorium
Thorium is one of only a few naturally radioactive elements that has a colossally long half-life, over 14 billion years. Despite its natural radioactivity, thorium has yet to be used as fuel in a commercially functional nuclear power plant, instead of being used in several small, niche applications.
Several countries have exerted some efforts in the direction of thorium-based nuclear power, mostly because a large amount of thorium is available, and because it is extremely hard to convert any products from spent thorium fuel into a weapons-grade fissile material. India may be the first country to harness thorium to this purpose, in large part because India has large deposits of thorium-containing minerals (USGS, 2013; The International Thorium Energy Organization, 2014).
19.1 Sources
Monazite is a mineral that can not only be considered a major source of the element thorium, but it also always contains some lanthanide elements. Because thorium is not used in large quantities compared to many other industrially important metallic elements, the United States Geologic Survey tracks it in terms of reserves of thorium oxide (ThO2), and does not list refinery production (USGS, 2013). This thorium oxide, or thoria, production is often a secondary product with rare earth elements being the primary product.
Figure 19.1 shows the current world thorium reserves of different countries, measured in terms of metric tons of ThO2. One can see that the total world reserve is only 1.4 million tons, which is much less than several other industrially useful metals.
A word of caution is in order when discussing thorium reserves: these estimates may change significantly in coming years. Currently, there is not a high demand for thorium. Should thorium-based nuclear reactors become an important way to produce electricity in coming decades, there will be much more intense exploration for monazite and thus for thorium deposits (The International Thorium Energy Organization, 2014; Martin and Richard, 2013; Greentech Media, 2014).
19.2 Extraction chemistry
The extraction processes for thorium are relatively complex because thorium always occurs in conjunction with several of the lanthanide elements. As mentioned, monazite is the mineral from which thorium is generally refined although thorite contains more thorium by the mass percentage of mineral. But monazite is also a mineral from which several rare earth elements are refined, as discussed in Chapter 18. Usually, refining of the rare earth elements is the economic driving force for the extraction of materials from monazite. The United States Geological Survey Mineral Commodity Summaries 2013 states:
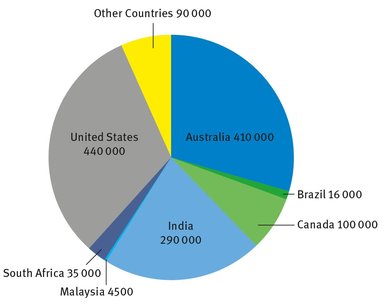
Fig. 19.1: World thorium reserves.
Reserves are contained primarily in the rare-earth ore mineral monazite and the thorium mineral thorite. Without demand for the rare earths, monazite would probably not be recovered for its thorium content. Other ore materials with higher thorium contents, such as thorite, would be more likely sources if demand significantly increased (USGS, 2013).
A very simplified chemistry for the isolation of thorium from a lanthanide-containing ore is shown in Figure 19.2.
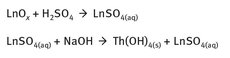
Fig. 19.2: Thorium isolation.
In the first equation, Ln represents the mix of lanthanide elements and thorium, and the reaction must be heated to produce a solution of lanthanide—thorium sulfates. In the second reaction, the reaction conditions must be adjusted to a pH range of 3—4 so that the resulting thorium hydroxide will precipitate from the solution.
Table 19.1: Uses for thorium or thorium compounds.
Form of thorium |
Uses |
Th metal |
Magnesium—thorium alloys for aircraft or rocket components (Mg—Th) |
ThF4 |
Antireflecting material in multilayered optics |
ThO2 (thoria) |
Ammonia → nitric acid catalyst |
ThO2 |
Ceramics, high temperature |
ThO2 |
Glass additive, for high precision lenses |
ThO2 |
Gas lamps, camping lantern mantels |
ThO2 |
Gas tungsten arc welding |
19.3 Uses
While energy production remains a potential use for thorium, it is currently also used in a variety of other ways, including: “catalysts, high temperature ceramics, and welding electrodes” (USGS, 2013). These are all smaller, niche applications, but they do have the potential for growth. Table 19.1 gives a summary of other uses for refined thorium, as well as for thorium-containing materials.
Thorium tetrafluoride is prepared from the direct combination of the metal with fluorine gas. Thoria is often a by-product of other extraction chemistry, but it becomes a useful material because of its very high melting point.
19.4 Potential uses for power
All the existing nuclear power plants throughout the world function with enriched uranium (235U) as fuel. Some have claimed that the reason for this is simply because the different governments that worked on nuclear weapons during the Second World War assembled teams of scientists and engineers who realized that the same enriched fuel, at a slightly lower level of enrichment, could be used to heat water, turn turbines, and thus produce electrical power from uranium’s nuclear decay (The International Thorium Energy Organization, 2014; Martin and Richard, 2013). These same sources usually point out that thorium is the more plentiful of the two actinides, and that it is difficult for thorium to produce plutonium — an element used in nuclear weaponry that is produced through beta-particle emission from uranium. The production of plutonium from uranium, usually 238U, is not a single-step process, but it does occur in fuel rods rather regularly. It involves a neutron absorption and two beta-particle emissions. The reaction chemistry can be represented in a straightforward manner, as shown in Figure 19.3.
![]()
Fig. 19.3: Plutonium production.
The use of thorium as fuel for nuclear power generation would in theory eliminate this transmutation pathway, and thus make the waste material from reactors much safer than that which currently exists.
19.5 Recycling
Thorium end uses in consumer materials mean that it is widely distributed into small items in such a manner that there is currently no financial need to recycle thorium. Should thorium be used as a fuel in any future nuclear reactor, disposal plans will have to be formulated for the fuel when it nears the end of its usable lifetime.
Bibliography
Greentech Media. Thorium Reactors: Nuclear Redemption or Nuclear Hazard. Website. (Accessed 29 May 2014 as: http://www.greentechmedia.com/articles/read/Thorium-Reactors-Nuclear-Redemption-or-Nuclear-Hazard).
The International Thorium Energy Organization. Website. (Accessed 29 May 2014, as: http://www.itheo.org).
Martin, Richard. SuperFuel: Thorium, the Green Energy Source for the Future, 2013, ISBN: 978-1137278340.
USGS Mineral Commodities Summary 2013. Website. (Accessed 20 May, 2014, as: http://minerals.usgs.gov/minerals/pubs/mcs/2013/mcs2013.pdf).