Industrial Chemistry: For Advanced Students - Mark A. Benvenuto 2015
Catalysts
Practically every general chemistry book used in secondary schools or colleges gives some space to the basic principles of catalysis. Such discussions always include the idea that a catalyst must speed up a reaction, and at the same time not be consumed in the reaction. Catalysts are generally divided into two categories: heterogeneous and homogeneous. Heterogeneous catalysts are not in the same phase as the reaction with which they interact, while homogeneous catalysts are. Less discussed are the costs of the catalyst itself, the ease of use, or the ease of recovery.
Despite a wide variety of catalysts that exist, or perhaps because of it, there is no single theory that encompasses why all catalysts function. Almost always, the use of a catalyst is an empirical decision, one that helps a reaction proceed. The field of catalyst chemistry is so wide that there are several societies devoted to it (The International Association of Catalysis Societies, 2014; Acmite Market Intelligence, 2014) which continue to examine mature as well as new reactions for possibilities concerning catalyst use.
20.1 Uses
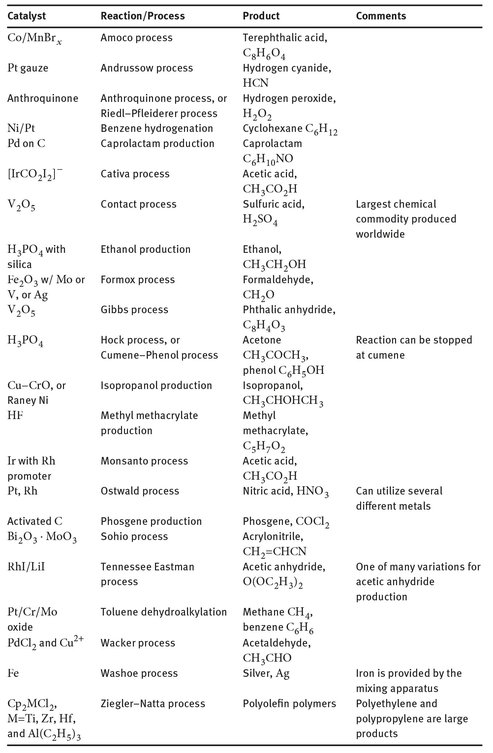
Since there is no overarching theory for the production and application of catalysts, it is probably useful to simply tabulate several more industrially useful catalysts. Table 20.1 is a nonexhaustive list that shows many of the common catalysts used in different sectors of the chemical industry and the reactions with which they are associated. The organization is simply an alphabetical one, based on the name of the reaction or process.
20.2 Syntheses
The simpler catalysts shown in Table 20.1 are materials which are themselves made in large quantities. There are however some catalysts that are produced much like fine chemicals. These may cost more than other catalysts, but the cost savings in making the bulk chemical with which they are associated makes the cost of the actual catalyst more affordable. Also, there is an enormous number of catalysts that have been proven useful for a reaction or class of reactions that have not been utilized for one of the industrial scale reactions discussed in this book (Strem Chemicals, 2014).
Table 20.1: Industrial processes and reactions incorporating catalyst.
20.2.1 Metallocenes
The metallocene catalysts can be categorized as those which must be synthesized, as opposed to refined or purified from some natural source. A sample reaction whereby a metallocene catalyst is formed is shown in Figure 20.1.
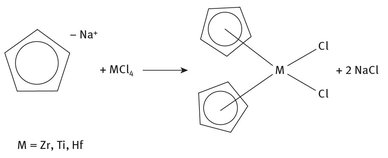
Fig. 20.1: Production of Metallocene Catalysts.
The number of metallocene catalysts is great, with different metal centers, and different organic ring components. The organic fraction shown in Figure 20.1, cyclopentadiene (abbreviated Cp), is usually refined and produced ultimately as part of petroleum distillation, but a wide variety of substituted Cp molecules must oftentimes themselves be synthesized. The success of Ziegler—Natta catalysis has fueled numerous research efforts in the past decades into reactions for which these catalysts might find some applicability.
20.2.2 Zeolites
This class of aluminosilicate materials is often used as catalysts for specific reactions, and usually they take advantage of a large surface area on which substrate molecules can react. But in the past 30 years, numerous zeolites have been manufactured. This is often done in what is called a sol—gel system, in which crystalline surfaces are grown slowly under precise pH conditions. The solution portion — the sol — is often a colloidal solution, and the final material is in the gel phase.
20.3 Recycling
Catalyst recycling is not something that involves an end-use product. Rather, the cost of a specific catalyst is usually enough that it is economically feasible to try to recover it from any end product, be it a plastic, or a bulk commodity, such as nitric acid. Table 20.1 illustrates how many expensive metals are used in different reactions, all of which are recovered from their reaction systems when possible.
Bibliography
Acmite Market Intelligence: http://www.acmite.com/market-reports/chemicals/global-catalyst-market-report.html. Website. (Accessed 2 June 2014, as: http://www.acmite.com/market-reports/chemicals/global-catalyst-market-report.html).
The International Association of Catalysis Societies. Website. (Accessed 2 June 2014, as: http://www.iacs-icc.org).
Strem Chemicals. Website. (Accessed 2 June 2014, as: http://www.strem.com/uploads/resources/documents/mcos_orgcat.pdf