Introductory Chemistry: A Foundation - Zumdahl S.S., DeCoste D.J. 2019
Measurements and Calculations
Temperature Conversions: An Approach to Problem Solving
Objectives
· To learn the three temperature scales.
· To learn to convert from one scale to another.
· To continue to develop problem-solving skills.
When the doctor tells you your temperature is degrees and the weatherperson on TV says it will be degrees tomorrow, they are using the Fahrenheit scale . Water boils at and freezes at , and normal body temperature is (where signifies “Fahrenheit degrees”). This temperature scale is widely used in the United States and Great Britain, and it is the scale employed in most of the engineering sciences. Another temperature scale, used in Canada and Europe and in the physical and life sciences in most countries, is the Celsius scale . In keeping with the metric system, which is based on powers of , the freezing and boiling points of water on the Celsius scale are assigned as and , respectively. On both the Fahrenheit and the Celsius scales, the unit of temperature is called a degree, and the symbol for it is followed by the capital letter representing the scale on which the units are measured: or .
Still another temperature scale used in the sciences is the absolute or Kelvin scale . On this scale water freezes at K and boils at K. On the Kelvin scale, the unit of temperature is called a kelvin and is symbolized by K. Thus, on the three scales, the boiling point of water is stated as Fahrenheit degrees , Celsius degrees , and kelvins ( K).
The three temperature scales are compared in Figs. 2.6 and 2.7. There are several important facts you should note.
Figure 2.6.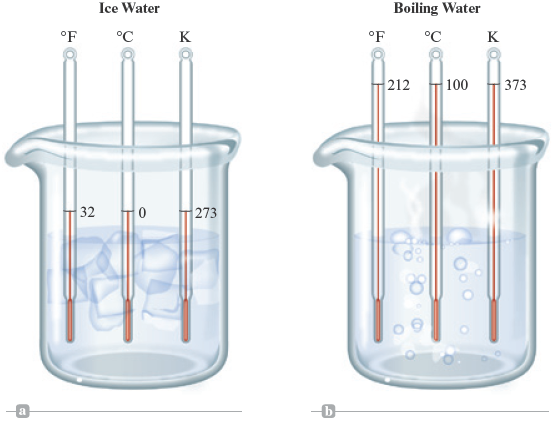
Thermometers based on the three temperature scales in (a) ice water and (b) boiling water.
Figure 2.7.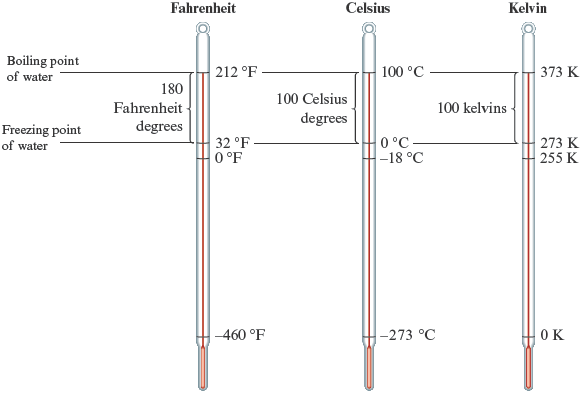
The three major temperature scales.
1. The size of each temperature unit (each degree) is the same for the Celsius and Kelvin scales. This follows from the fact that the difference between the boiling and freezing points of water is units on both of these scales.
2. The Fahrenheit degree is smaller than the Celsius and Kelvin units. Note that on the Fahrenheit scale there are Fahrenheit degrees between the boiling and freezing points of water compared with units on the other two scales.
3. The zero points are different on all three scales.
In your study of chemistry, you will sometimes need to convert from one temperature scale to another. We will consider in some detail how this is done. In addition to learning how to change temperature scales, you should also use this section as an opportunity to further develop your skills in problem solving.
Converting between the Kelvin and Celsius Scales
It is relatively simple to convert between the Celsius and Kelvin scales because the temperature unit is the same size; only the zero points are different. Because corresponds to K, converting from Celsius to Kelvin requires that we add to the Celsius temperature. We will illustrate this procedure in Example 2.8.
Interactive Example 2.8. Temperature Conversion: Celsius to Kelvin
The boiling point of water at the top of Mt. Everest is . Convert this temperature to the Kelvin scale. (The decimal point after the temperature reading indicates that the trailing zero is significant.)
Solution
This problem asks us to find in units of kelvins. We can represent this problem simply as
In doing problems, it is often helpful to draw a diagram in which we try to represent the words in the problem with a picture. This problem can be diagramed as shown in Fig. 2.8(a).
Figure 2.8.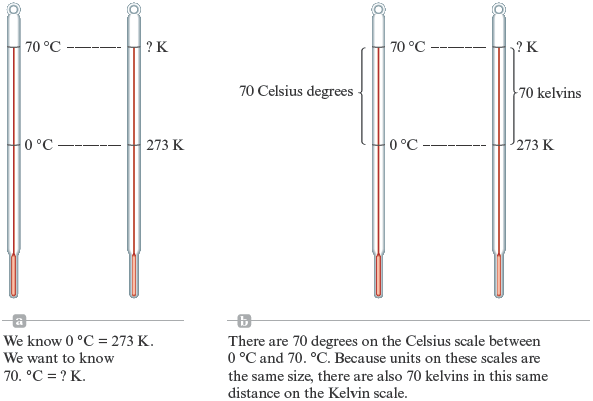
Converting to units measured on the Kelvin scale.
In this picture we have shown what we want to find: “What temperature (in kelvins) is the same as ?” We also know from Fig. 2.7 that represents the same temperature as K. How many degrees above is ? The answer, of course, is . Thus we must add . to to reach . Because degrees are the same size on both the Celsius scale and the Kelvin scale [Fig. 2.8(b)], we must also add . to K (same temperature as ) to reach ? K. That is,
Thus corresponds to K.
Note that to convert from the Celsius to the Kelvin scale, we simply add the temperature in to . That is,
Using this formula to solve the present problem gives
(with units of kelvins, K), which is the correct answer.
We can summarize what we learned in Example 2.8 as follows: to convert from the Celsius to the Kelvin scale, we can use the formula
Interactive Example 2.9. Temperature Conversion: Kelvin to Celsius
Liquid nitrogen boils at K. What is the boiling point of nitrogen on the Celsius scale?
Solution
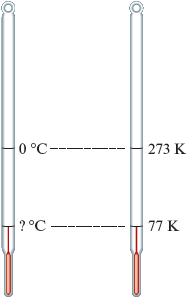
The problem to be solved here is . Let’s explore this question by examining the picture to the left representing the two temperature scales. One key point is to recognize that . Also note that the difference between K and K is . That is, K is kelvins below K. The degree size is the same on these two temperature scales, so K must correspond to Celsius degrees below zero or . Thus .
We can also solve this problem by using the formula
However, in this case we want to solve for the Celsius temperature, . That is, we want to isolate on one side of the equals sign. To do this we use an important general principle: doing the same thing on both sides of the equals sign preserves the equality. In other words, it’s always okay to perform the same operation on both sides of the equals sign.
To isolate we need to subtract from both sides:
to give
Using this equation to solve the problem, we have
So, as before, we have shown that
Self-Check: Exercise 2.6
· Which temperature is colder, K or
See Problems 2.73 and 2.74.
Chemistry in Focus Tiny Thermometers
Can you imagine a thermometer that has a diameter equal to one one-hundredth of a human hair? Such a device has actually been produced by scientists Yihua Gao and Yoshio Bando of the National Institute for Materials Science in Tsukuba, Japan. The thermometer they constructed is so tiny that it must be read using a powerful electron microscope.
It turns out that the tiny thermometers were produced by accident. The Japanese scientists were actually trying to make tiny (nanoscale) gallium nitride wires. However, when they examined the results of their experiment, they discovered tiny tubes of carbon atoms that were filled with elemental gallium. Because gallium is a liquid over an unusually large temperature range, it makes a perfect working liquid for a thermometer. Just as in mercury thermometers, which have mostly been phased out because of the toxicity of mercury, the gallium expands as the temperature increases. Therefore, gallium moves up the tube as the temperature increases.
These minuscule thermometers are not useful in the normal macroscopic world—they can’t even be seen with the naked eye. However, they should be valuable for monitoring temperatures from to in materials in the nanoscale world.
See Problem 2.77
In summary, because the Kelvin and Celsius scales have the same size unit, to switch from one scale to the other we must simply account for the different zero points. We must add to the Celsius temperature to obtain the temperature on the Kelvin scale:
To convert from the Kelvin scale to the Celsius scale, we must subtract from the Kelvin temperature:
Converting between the Fahrenheit and Celsius Scales
The conversion between the Fahrenheit and Celsius temperature scales requires two adjustments:
1. For the different size units
2. For the different zero points
To see how to adjust for the different unit sizes, consider the diagram in Fig. 2.9. Note that because and ,
Figure 2.9.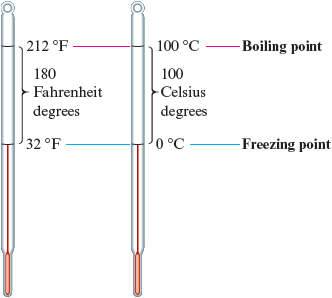
Comparison of the Celsius and Fahrenheit scales.
Thus
Dividing both sides of this equation by gives
or
The factor is used to convert from one degree size to the other.
Next we have to account for the fact that is not the same as . In fact, . Although we will not show how to derive it, the equation to convert a temperature in Celsius degrees to the Fahrenheit scale is
In this equation the term adjusts for the difference in degree size between the two scales. The in the equation accounts for the different zero points. We will now show how to use this equation.
Interactive Example 2.10. Temperature Conversion: Celsius to Fahrenheit
On a summer day the temperature in the laboratory, as measured on a lab thermometer, is . Express this temperature on the Fahrenheit scale.
Solution
This problem can be represented as . We will solve it using the formula
In this case,
Thus .
Interactive Example 2.11. Temperature Conversion: Celsius to Fahrenheit
Express the temperature on the Fahrenheit scale.
Solution
We can express this problem as . To solve it we will use the formula
In this case,
So . This is a very interesting result and is another useful reference point.
Self-Check: Exercise 2.7
· Hot tubs are often maintained at . What is this temperature in Fahrenheit degrees?
See Problems 2.75, 2.76, 2.77, and 2.78.
To convert from Celsius to Fahrenheit, we have used the equation
To convert a Fahrenheit temperature to Celsius, we need to rearrange this equation to isolate Celsius degrees . Remember, we can always do the same operation to both sides of the equation. First subtract from each side:
to give
Next divide both sides by
to give
or

Interactive Example 2.12. Temperature Conversion: Fahrenheit to Celsius
One of the body’s responses to an infection or injury is to elevate its temperature. A certain flu victim has a body temperature of . What is this temperature on the Celsius scale?
Solution
The problem is . Using the formula
yields
That is, .
Self-Check: Exercise 2.8
· An antifreeze solution in a car’s radiator boils at . What is this temperature on the Celsius scale?
See Problems 2.75, 2.76, 2.77, and, 2.78.
In doing temperature conversions, you will need the following formulas.
Temperature Conversion Formulas
· Celsius to Kelvin
· Kelvin to Celsius
· Celsius to Fahrenheit
· Fahrenheit to Celsius