Introductory Chemistry: A Foundation - Zumdahl S.S., DeCoste D.J. 2019
Chemical Foundations: Elements, Atoms, and Ions
Natural States of the Elements
Objective
· To learn the natures of the common elements.
As we have noted, the matter around us consists mainly of mixtures. Most often these mixtures contain compounds, in which atoms from different elements are bound together. Most elements are quite reactive: their atoms tend to combine with those of other elements to form compounds. Thus we do not often find elements in nature in pure form—uncombined with other elements. However, there are notable exceptions. The gold nuggets found at Sutter’s Mill in California that launched the Gold Rush in 1849 are virtually pure elemental gold. And platinum and silver are often found in nearly pure form.
Gold, silver, and platinum are members of a class of metals called noble metals because they are relatively unreactive. (The term noble implies a class set apart.)
Other elements that appear in nature in the uncombined state are the elements in Group 8: helium, neon, argon, krypton, xenon, and radon. Because the atoms of these elements do not combine readily with those of other elements, we call them the noble gases. For example, helium gas is found in uncombined form in underground deposits with natural gas.
When we take a sample of air (the mixture of gases that constitute the earth’s atmosphere) and separate it into its components, we find several pure elements present. One of these is argon. Argon gas consists of a collection of separate argon atoms, as shown in Fig. 4.11.
Figure 4.11.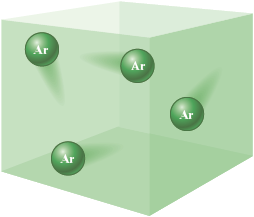
Argon gas consists of a collection of separate argon atoms.
Air also contains nitrogen gas and oxygen gas. When we examine these two gases, however, we find that they do not contain single atoms, as argon does, but instead contain diatomic molecules : molecules made up of two atoms, as represented in Fig. 4.12. In fact, any sample of elemental oxygen gas at normal temperatures contains molecules. Likewise, nitrogen gas contains molecules.
Figure 4.12.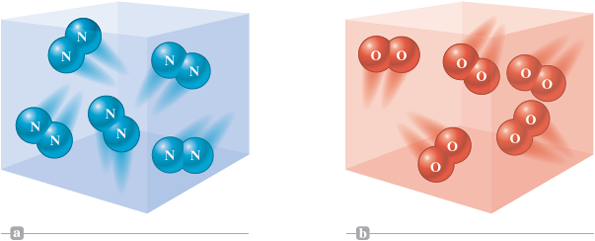
Gaseous nitrogen and oxygen contain diatomic (two-atom) molecules.
Hydrogen is another element that forms diatomic molecules. Although virtually all of the hydrogen found on earth is present in compounds with other elements (such as with oxygen in water), when hydrogen is prepared as a free element, it contains diatomic molecules. For example, an electric current can be used to decompose water (Fig. 4.13 and Fig. 3.3) into elemental hydrogen and oxygen containing and molecules, respectively.
Figure 4.13.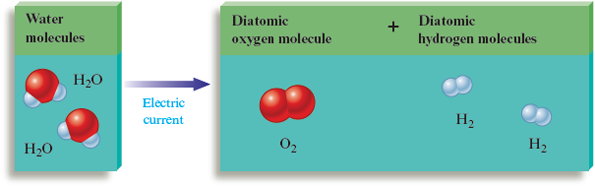
The decomposition of two water molecules to form two hydrogen molecules and one oxygen molecule . Note that only the grouping of the atoms changes in this process; no atoms are created or destroyed. There must be the same number of atoms and atoms before and after the process. Thus the decomposition of two molecules (containing four atoms and two atoms) yields one molecule (containing two atoms) and two molecules (containing a total of four atoms).
Several other elements, in addition to hydrogen, nitrogen, and oxygen, exist as diatomic molecules. For example, when sodium chloride is melted and subjected to an electric current, chlorine gas is produced (along with sodium metal). This chemical change is represented in Fig. 4.14. Chlorine gas is a pale green gas that contains molecules.
Figure 4.14.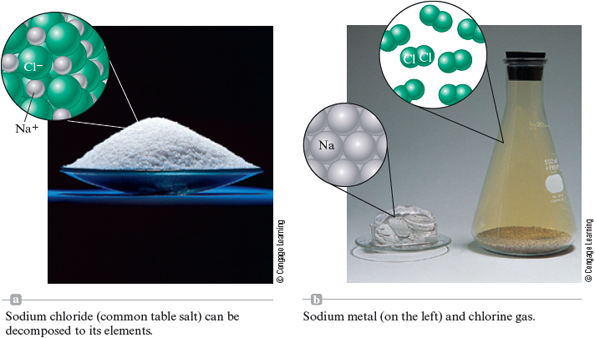
© Cengage Learning
Chlorine is a member of Group 7, the halogen family. All the elemental forms of the Group 7 elements contain diatomic molecules. Fluorine is a pale yellow gas containing molecules. Bromine is a brown liquid made up of molecules. Iodine is a lustrous, purple solid that contains molecules.
Table 4.5 lists the elements that contain diatomic molecules in their pure, elemental forms.
Table 4.5. Elements That Exist as Diatomic Molecules in Their Elemental Forms
Element Present |
Elemental State at |
Molecule |
hydrogen |
colorless gas |
|
nitrogen |
colorless gas |
|
oxygen |
pale blue gas |
|
fluorine |
pale yellow gas |
|
chlorine |
pale green gas |
|
bromine |
reddish-brown liquid |
|
iodine |
lustrous, dark purple solid |
So far we have seen that several elements are gaseous in their elemental forms at normal temperatures . The noble gases (the Group 8 elements) contain individual atoms, whereas several other gaseous elements contain diatomic molecules ( , , , , and ).
Only two elements are liquids in their elemental forms at : the nonmetal bromine (containing molecules) and the metal mercury. The metals gallium and cesium almost qualify in this category; they are solids at , but both melt at .

Harry Taylor/Dorling Kindersley/Getty Images
Platinum is a noble metal used in jewelry and in many industrial processes.
The other elements are solids in their elemental forms at . For metals these solids contain large numbers of atoms packed together much like marbles in a jar (Fig. 4.15).

© Cengage Learning
Liquid bromine in a flask with bromine vapor.
Figure 4.15.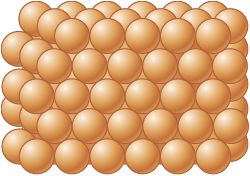
In solid metals, the spherical atoms are packed closely together.
The structures of solid nonmetallic elements are more varied than those of metals. In fact, different forms of the same element often occur. For example, solid carbon occurs in three forms. Different forms of a given element are called allotropes. The three allotropes of carbon are the familiar diamond and graphite forms plus a form that has only recently been discovered called buckminsterfullerene. These elemental forms have very different properties because of their different structures (Fig. 4.16). Diamond is the hardest natural substance known and is often used for industrial cutting tools. Diamonds are also valued as gemstones. Graphite, by contrast, is a rather soft material useful for writing (pencil “lead” is really graphite) and (in the form of a powder) for lubricating locks. The rather odd name given to buckminsterfullerene comes from the structure of the molecules that form this allotrope. The soccer-ball-like structure contains five- and six-member rings reminiscent of the structure of geodesic domes suggested by the late industrial designer Buckminster Fuller. Other “fullerenes” containing molecules with more than carbon atoms have also been discovered, leading to a new area of chemistry.
Figure 4.16.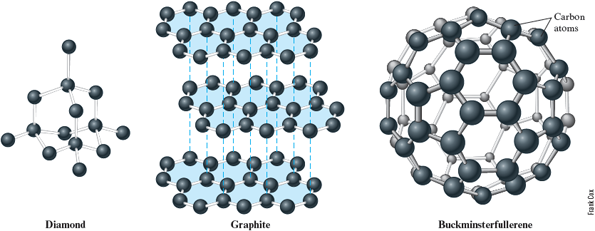
Frank Cox
The three solid elemental (allotropes) forms of carbon. The representations of diamond and graphite are just fragments of much larger structures that extend in all directions from the parts shown here. Buckminsterfullerene contains molecules, one of which is shown.