Introductory Chemistry: A Foundation - Zumdahl S.S., DeCoste D.J. 2019
Chemical Foundations: Elements, Atoms, and Ions
Ions
Objectives
· To understand the formation of ions from their parent atoms, and learn to name them.
· To learn how the periodic table can help predict which ion a given element forms.
We have seen that an atom has a certain number of protons in its nucleus and an equal number of electrons in the space around the nucleus. This results in an exact balance of positive and negative charges. We say that an atom is a neutral entity—it has zero net charge.
We can produce a charged entity, called an ion , by taking a neutral atom and adding or removing one or more electrons. For example, a sodium atom has protons in its nucleus and electrons outside its nucleus.
If one of the electrons is lost, there will be positive charges but only negative charges. This gives an ion with a net positive one charge: . We can represent this process as follows:
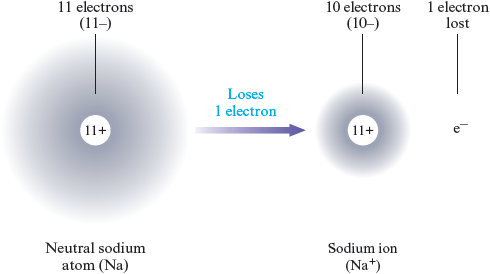
or, in shorthand form, as
where represents the neutral sodium atom, represents the ion formed, and represents an electron.
A positive ion, called a cation (pronounced cat’ eye on), is produced when one or more electrons are lost from a neutral atom. We have seen that sodium loses one electron to become a cation. Some atoms lose more than one electron. For example, a magnesium atom typically loses two electrons to form a cation:
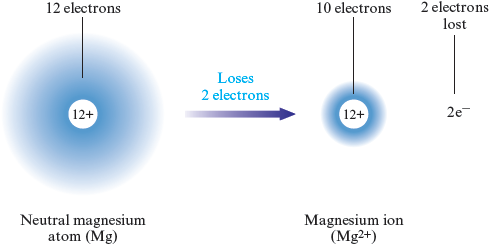
We usually represent this process as follows:
Aluminum forms a cation by losing three electrons:
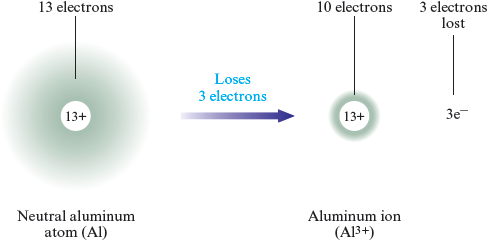
or
A cation is named using the name of the parent atom. Thus is called the sodium ion (or sodium cation), is called the magnesium ion (or magnesium cation), and is called the aluminum ion (or aluminum cation).
When electrons are gained by a neutral atom, an ion with a negative charge is formed. A negatively charged ion is called an anion (pronounced an’ ion). An atom that gains one extra electron forms an anion with a charge. An example of an atom that forms a anion is the chlorine atom, which has protons and electrons:
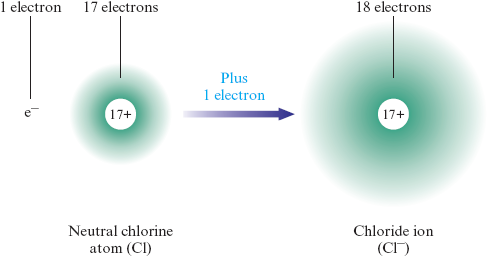
This process is usually represented as
Note that the anion formed by chlorine has electrons but only protons, so the net charge is . Unlike a cation, which is named for the parent atom, an anion is named by taking the root name of the atom and changing the ending. For example, the anion produced from the (chlorine) atom is called the chloride ion (or chloride anion). Notice that the word chloride is obtained from the root of the atom name (chlor-) plus the suffix -ide. Other atoms that add one electron to form ions include
fluorine |
(fluoride ion) |
|
bromine |
(bromide ion) |
|
iodine |
(iodide ion) |
Note that the name of each of these anions is obtained by adding -ide to the root of the atom name.
Some atoms can add two electrons to form anions. Examples include
oxygen |
(oxide ion) |
|
sulfur |
(sulfide ion) |
Note that the names for these anions are derived in the same way as those for the anions.
It is important to recognize that ions are always formed by removing electrons from an atom (to form cations) or adding electrons to an atom (to form anions). Ions are never formed by changing the number of protons in an atom’s nucleus.
It is essential to understand that isolated atoms do not form ions on their own. Most commonly, ions are formed when metallic elements combine with nonmetallic elements. As we will discuss in detail in Chapter 7, when metals and nonmetals react, the metal atoms tend to lose one or more electrons, which are in turn gained by the atoms of the nonmetal. Thus reactions between metals and nonmetals tend to form compounds that contain metal cations and nonmetal anions. We will have more to say about these compounds in Section 4.11.
Ion Charges and the Periodic Table
We find the periodic table very useful when we want to know what type of ion is formed by a given atom. Fig. 4.17 shows the types of ions formed by atoms in several of the groups on the periodic table. Note that the Group 1 metals all form ions , the Group 2 metals all form ions , and the Group 3 metals form ions . Thus for Groups 1 through 3 the charges of the cations formed are identical to the group numbers.
Figure 4.17.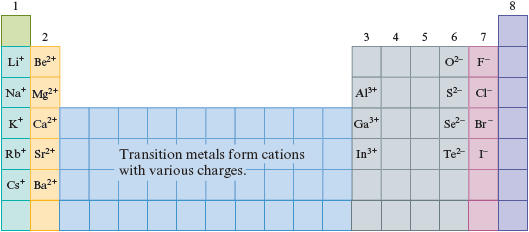
The ions formed by selected members of Groups 1, 2, 3, 6, and 7.
In contrast to the Group 1, 2, and 3 metals, most of the many transition metals form cations with various positive charges. For these elements there is no easy way to predict the charge of the cation that will be formed.
Note that metals always form positive ions. This tendency to lose electrons is a fundamental characteristic of metals. Nonmetals, on the other hand, form negative ions by gaining electrons. Note that the Group 7 atoms all gain one electron to form ions and that all the nonmetals in Group 6 gain two electrons to form ions.
At this point you should memorize the relationships between the group number and the type of ion formed, as shown in Fig. 4.17. You will understand why these relationships exist after we further discuss the theory of the atom in Chapter 11.