Introductory Chemistry: A Foundation - Zumdahl S.S., DeCoste D.J. 2019
Chemical Reactions: An Introduction
Chemical Equations
Objective
· To learn to identify the characteristics of a chemical reaction and the information given by a chemical equation.
Chemists have learned that a chemical change always involves a rearrangement of the ways in which the atoms are grouped. For example, when the methane, , in natural gas combines with oxygen, , in the air and burns, carbon dioxide, , and water, , are formed. A chemical change such as this is called a chemical reaction . We represent a chemical reaction by writing a chemical equation in which the chemicals present before the reaction (the reactants ) are shown to the left of an arrow and the chemicals formed by the reaction (the products ) are shown to the right of an arrow. The arrow indicates the direction of the change and is read as “yields” or “produces”:
For the reaction of methane with oxygen, we have
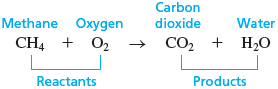
Note from this equation that the products contain the same atoms as the reactants but that the atoms are associated in different ways. That is, a chemical reaction involves changing the ways the atoms are grouped.
It is important to recognize that in a chemical reaction, atoms are neither created nor destroyed. All atoms present in the reactants must be accounted for among the products. In other words, there must be the same number of each type of atom on the product side as on the reactant side of the arrow. Making sure that the equation for a reaction obeys this rule is called balancing the chemical equation for a reaction.
The equation that we have shown for the reaction between and is not balanced. We can see that it is not balanced by taking the reactants and products apart.
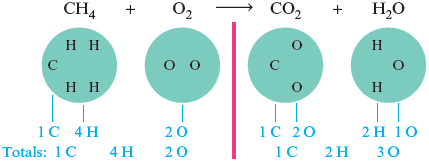
The reaction cannot happen this way because, as it stands, this equation states that one oxygen atom is created and two hydrogen atoms are destroyed. A reaction is only a rearrangement of the way the atoms are grouped; atoms are not created or destroyed. The total number of each type of atom must be the same on both sides of the arrow. We can fix the imbalance in this equation by involving one more molecule on the left and by showing the production of one more molecule on the right.
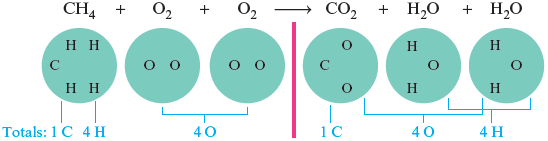
This balanced chemical equation shows the actual numbers of molecules involved in this reaction (Fig. 6.4).
Figure 6.4.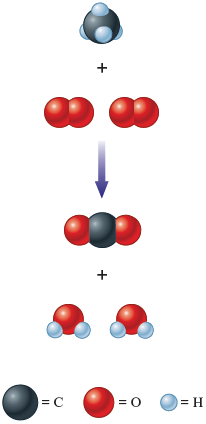
The reaction between methane and oxygen to give water and carbon dioxide. Note that there are four oxygen atoms in the products and in the reactants; none has been gained or lost in the reaction. Similarly, there are four hydrogen atoms and one carbon atom in the reactants and in the products. The reaction simply changes the way the atoms are grouped.
When we write the balanced equation for a reaction, we group like molecules together. Thus
is written
The chemical equation for a reaction provides us with two important types of information:
1. The identities of the reactants and products
2. The relative numbers of each
Physical States
Besides specifying the compounds involved in the reaction, we often indicate in the equation the physical states of the reactants and products by using the following symbols:
Symbol |
State |
solid |
|
liquid |
|
gas |
|
dissolved in water (in aqueous solution) |
For example, when solid potassium reacts with liquid water, the products are hydrogen gas and potassium hydroxide; the latter remains dissolved in the water. From this information about the reactants and products, we can write the equation for the reaction. Solid potassium is represented by ; liquid water is written as ; hydrogen gas contains diatomic molecules and is represented as ; potassium hydroxide dissolved in water is written as . So the unbalanced equation for the reaction is
This reaction is shown in Fig. 6.5.
Figure 6.5.
Richard Megna/Fundamental Photographs © Cengage Learning
The hydrogen gas produced in this reaction then reacts with the oxygen gas in the air, producing gaseous water and a flame. The unbalanced equation for this second reaction is
Both of these reactions produce a great deal of heat. In Example 6.1 we will practice writing the unbalanced equations for reactions. Then, in the next section, we will discuss systematic procedures for balancing equations.
Example 6.1. Chemical Equations: Recognizing Reactants and Products
Write the unbalanced chemical equation for each of the following reactions.
a. Solid mercury(II) oxide decomposes to produce liquid mercury metal and gaseous oxygen.
b. Solid carbon reacts with gaseous oxygen to form gaseous carbon dioxide.
c. Solid zinc is added to an aqueous solution containing dissolved hydrogen chloride to produce gaseous hydrogen that bubbles out of the solution and zinc chloride that remains dissolved in the water.
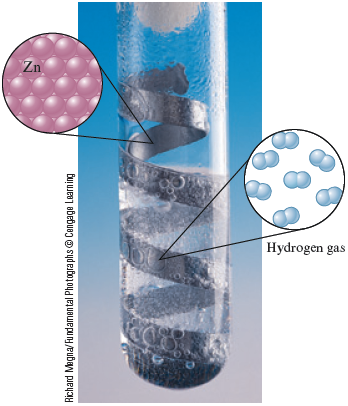
Richard Megna/Fundamental Photographs © Cengage Learning
Zinc metal reacts with hydrochloric acid to produce bubbles of hydrogen gas.
Solution
a. In this case we have only one reactant, mercury(II) oxide. The name mercury(II) oxide means that the cation is present, so one ion is required for a zero net charge. Thus the formula is , which is written in this case because it is given as a solid. The products are liquid mercury, written , and gaseous oxygen, written . (Remember that oxygen exists as a diatomic molecule under normal conditions.) The unbalanced equation is
b. In this case, solid carbon, written , reacts with oxygen gas, , to form gaseous carbon dioxide, which is written . The equation (which happens to be balanced) is
c. In this reaction solid zinc, , is added to an aqueous solution of hydrogen chloride, which is written and called hydrochloric acid. These are the reactants. The products of the reaction are gaseous hydrogen, , and aqueous zinc chloride. The name zinc chloride means that the ion is present, so two ions are needed to achieve a zero net charge. Thus zinc chloride dissolved in water is written . The unbalanced equation for the reaction is
Self-Check: Exercise 6.1
· Identify the reactants and products and write the unbalanced equation (including symbols for states) for each of the following chemical reactions.
a. Solid magnesium metal reacts with liquid water to form solid magnesium hydroxide and hydrogen gas.
b. Solid ammonium dichromate (review Table 5.4 if this compound is unfamiliar) decomposes to solid chromium(III) oxide, gaseous nitrogen, and gaseous water.
c. Gaseous ammonia reacts with gaseous oxygen to form gaseous nitrogen monoxide and gaseous water.
See Problems 6.13, 6.14, 6.15, 6.16, 6.17, 6.18, 6.19, 6.20, 6.21, 6.22, 6.23, 6.24, 6.25, 6.26, 6.27, 6.28, 6.29, 6.30, 6.31, 6.32, 6.33, and 6.34.