Introductory Chemistry: A Foundation - Zumdahl S.S., DeCoste D.J. 2019
Chemical Reactions: An Introduction
Balancing Chemical Equations
Objective
· To learn how to write a balanced equation for a chemical reaction.
As we saw in the previous section, an unbalanced chemical equation is not an accurate representation of the reaction that occurs. Whenever you see an equation for a reaction, you should ask yourself whether it is balanced. The principle that lies at the heart of the balancing process is that atoms are conserved in a chemical reaction. That is, atoms are neither created nor destroyed. They are just grouped differently. The same number of each type of atom is found among the reactants and among the products.
Chemists determine the identity of the reactants and products of a reaction by experimental observation. For example, when methane (natural gas) is burned in the presence of sufficient oxygen gas, the products are always carbon dioxide and water. The identities (formulas) of the compounds must never be changed in balancing a chemical equation. In other words, the subscripts in a formula cannot be changed, nor can atoms be added to or subtracted from a formula.
Most chemical equations can be balanced by trial and error—that is, by inspection. Keep trying until you find the numbers of reactants and products that give the same number of each type of atom on both sides of the arrow. For example, consider the reaction of hydrogen gas and oxygen gas to form liquid water. First, we write the unbalanced equation from the description of the reaction.
We can see that this equation is unbalanced by counting the atoms on both sides of the arrow.
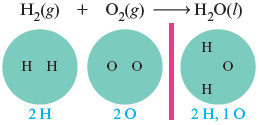
We have one more oxygen atom in the reactants than in the product. Because we cannot create or destroy atoms and because we cannot change the formulas of the reactants or products, we must balance the equation by adding more molecules of reactants and/or products. In this case we need one more oxygen atom on the right, so we add another water molecule (which contains one atom). Then we count all of the atoms again.
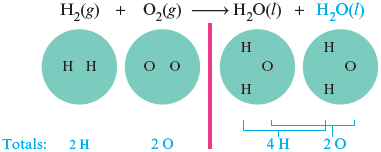
We have balanced the oxygen atoms, but now the hydrogen atoms have become unbalanced. There are more hydrogen atoms on the right than on the left. We can solve this problem by adding another hydrogen molecule to the reactant side.
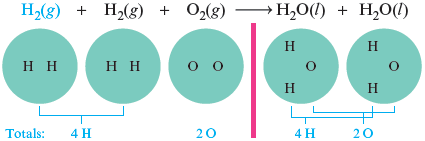
The equation is now balanced. We have the same numbers of hydrogen and oxygen atoms represented on both sides of the arrow. Collecting like molecules, we write the balanced equation as
Consider next what happens if we multiply every part of this balanced equation by :
to give
This equation is balanced (count the atoms to verify this). In fact, we can multiply or divide all parts of the original balanced equation by any number to give a new balanced equation. Thus each chemical reaction has many possible balanced equations. Is one of the many possibilities preferred over the others? Yes.
The accepted convention is that the “best” balanced equation is the one with the smallest integers (whole numbers). These integers are called the coefficients for the balanced equation. Therefore, for the reaction of hydrogen and oxygen to form water, the “correct” balanced equation is
The coefficients 2, 1 (never written), and 2, respectively, are the smallest integers that give a balanced equation for this reaction.
Critical Thinking
· What if a friend was balancing chemical equations by changing the values of the subscripts instead of using the coefficients? How would you explain to your friend that this tactic is the wrong approach?
Next we will balance the equation for the reaction of liquid ethanol, , with oxygen gas to form gaseous carbon dioxide and water. This reaction, among many others, occurs in engines that burn a gasoline—ethanol mixture called gasohol.
The first step in obtaining the balanced equation for a reaction is always to identify the reactants and products from the description given for the reaction. In this case we are told that liquid ethanol, , reacts with gaseous oxygen, , to produce gaseous carbon dioxide, , and gaseous water, . Therefore, the unbalanced equation is
Chemistry in Focus The Beetle That Shoots Straight
If someone said to you, “Name something that protects itself by spraying its enemies,” your answer would almost certainly be “a skunk.” Of course, you would be correct, but there is another correct answer—the bombardier beetle. When threatened, this beetle shoots a boiling stream of toxic chemicals at its enemy. How does this clever beetle accomplish this? Obviously, the boiling mixture cannot be stored inside the beetle’s body all the time. Instead, when endangered, the beetle mixes chemicals that produce the hot spray. The chemicals involved are stored in two compartments. One compartment contains the chemicals hydrogen peroxide and methylhydroquinone . The key reaction is the decomposition of hydrogen peroxide to form oxygen gas and water:
Hydrogen peroxide also reacts with the hydroquinones to produce other compounds that become part of the toxic spray.

Reuters Pictures
A bombardier beetle defending itself.
However, none of these reactions occurs very fast unless certain enzymes are present. (Enzymes are natural substances that speed up biological reactions by means we will not discuss here.) When the beetle mixes the hydrogen peroxide and hydroquinones with the enzyme, the decomposition of occurs rapidly, producing a hot mixture pressurized by the formation of oxygen gas. When the gas pressure becomes high enough, the hot spray is ejected in one long stream or in short bursts. The beetle has a highly accurate aim and can shoot several attackers with one batch of spray.
See Problem 6.36
When one molecule in an equation is more complicated (contains more elements) than the others, it is best to start with that molecule. The most complicated molecule here is , so we begin by considering the products that contain the atoms in . We start with carbon. The only product that contains carbon is . Because contains two carbon atoms, we place a before the to balance the carbon atoms.
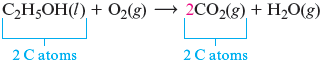
Remember, we cannot change the formula of any reactant or product when we balance an equation. We can only place coefficients in front of the formulas.
Next we consider hydrogen. The only product containing hydrogen is . contains six hydrogen atoms, so we need six hydrogen atoms on the right. Because each contains two hydrogen atoms, we need three molecules to yield six hydrogen atoms. So we place a before the .
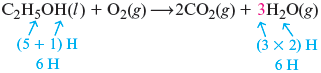
Finally, we count the oxygen atoms. On the left we have three oxygen atoms (one in and two in ), and on the right we have seven oxygen atoms (four in and three in ). We can correct this imbalance if we have three molecules on the left. That is, we place a coefficient of before the to produce the balanced equation.
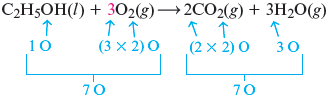
At this point you may have a question: why did we choose on the left when we balanced the oxygen atoms? Why not use , which has an oxygen atom? The answer is that if we had changed the coefficient in front of , we would have unbalanced the hydrogen and carbon atoms. Now we count all of the atoms as a check to make sure the equation is balanced.
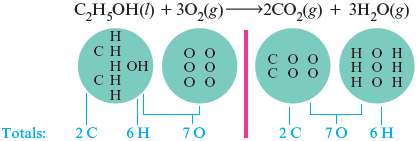
The equation is now balanced. We have the same numbers of all types of atoms on both sides of the arrow. Notice that these coefficients are the smallest integers that give a balanced equation.
The process of writing and balancing the equation for a chemical reaction consists of several steps:
How to Write and Balance Equations
Step 1.
Read the description of the chemical reaction. What are the reactants, the products, and their states? Write the appropriate formulas.
Step 2.
Write the unbalanced equation that summarizes the information from step 1.
Step 3.
Balance the equation by inspection, starting with the most complicated molecule. Proceed element by element to determine what coefficients are necessary so that the same number of each type of atom appears on both the reactant side and the product side. Do not change the identities (formulas) of any of the reactants or products.
Step 4.
Check to see that the coefficients used give the same number of each type of atom on both sides of the arrow. (Note that an “atom” may be present in an element, a compound, or an ion.) Also check to see that the coefficients used are the smallest integers that give the balanced equation. This can be done by determining whether all coefficients can be divided by the same integer to give a set of smaller integer coefficients.
Critical Thinking
· One part of the problem-solving strategy for balancing chemical equations is “starting with the most complicated molecule.” What if you started with a different molecule? Could you still eventually balance the chemical equation? How would this approach be different from the suggested technique?
Interactive Example 6.2. Balancing Chemical Equations I
For the following reaction, write the unbalanced equation and then balance the equation: solid potassium reacts with liquid water to form gaseous hydrogen and potassium hydroxide that dissolves in the water.
Solution
Step 1
From the description given for the reaction, we know that the reactants are solid potassium, , and liquid water, . The products are gaseous hydrogen, , and dissolved potassium hydroxide, .
Step 2
The unbalanced equation for the reaction is
Step 3
Although none of the reactants or products is very complicated, we will start with because it contains the most elements (three). We will arbitrarily consider hydrogen first. Note that on the reactant side of the equation in step 2, there are two hydrogen atoms, but on the product side, there are three. If we place a coefficient of in front of both and , we now have four atoms on each side.
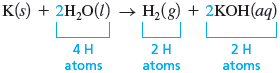
Also note that the oxygen atoms balance.
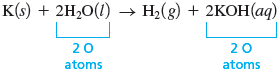
However, the atoms do not balance; we have one on the left and two on the right. We can fix this easily by placing a coefficient of in front of to give the balanced equation:
Step 4
Check There are , , and on both sides of the arrow, and the coefficients are the smallest integers that give a balanced equation. We know this because we cannot divide through by a given integer to give a set of smaller integer (whole-number) coefficients. For example, if we divide all of the coefficients by , we get
This is not acceptable because the coefficient for is not an integer.
Interactive Example 6.3. Balancing Chemical Equations II
Under appropriate conditions at , ammonia gas reacts with oxygen gas to produce gaseous nitrogen monoxide (common name, nitric oxide) and gaseous water. Write the unbalanced and balanced equations for this reaction.
Solution
Step 1
The reactants are gaseous ammonia, , and gaseous oxygen, . The products are gaseous nitrogen monoxide, , and gaseous water, .
Step 2
The unbalanced equation for the reaction is
Step 3
In this equation there is no molecule that is obviously the most complicated. Three molecules contain two elements, so we arbitrarily start with . We arbitrarily begin by looking at hydrogen. A coefficient of for and a coefficient of for give six atoms of hydrogen on both sides.
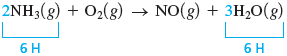
We can balance the nitrogen by giving a coefficient of .
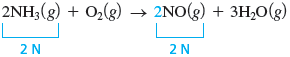
Finally, we note that there are two atoms of oxygen on the left and five on the right. The oxygen can be balanced with a coefficient of for , because gives five oxygen atoms.
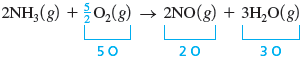
However, the convention is to have integer (whole-number) coefficients, so we multiply the entire equation by .
or
Step 4
Check There are , , and atoms on both sides, so the equation is balanced. These coefficients are the smallest integers that give a balanced equation. That is, we cannot divide all coefficients by the same integer and obtain a smaller set of integers.
Self-Check: Exercise 6.2
· Propane, , a liquid at under high pressure, is often used for gas grills and as a fuel in rural areas where there is no natural gas pipeline. When liquid propane is released from its storage tank, it changes to propane gas that reacts with oxygen gas (it “burns”) to give gaseous carbon dioxide and gaseous water. Write and balance the equation for this reaction.
Hint This description of a chemical process contains many words, some of which are crucial to solving the problem and some of which are not. First sort out the important information and use symbols to represent it.
See Problems 6.37, 6.38, 6.39, 6.40, 6.41, 6.42, 6.43, and 6.44.
Interactive Example 6.4. Balancing Chemical Equations III
Glass is sometimes decorated by etching patterns on its surface. Etching occurs when hydrofluoric acid (an aqueous solution of ) reacts with the silicon dioxide in the glass to form gaseous silicon tetrafluoride and liquid water. Write and balance the equation for this reaction.

Richard Megna/Fundamental Photographs © Cengage Learning
Decorations on glass are produced by etching with hydrofluoric acid.
Solution
Step 1
From the description of the reaction we can identify the reactants:
hydrofluoric acid |
|
solid silicon dioxide |
and the products:
gaseous silicon tetrafluoride |
|
liquid water |
Step 2
The unbalanced equation is
Step 3
There is no clear choice here for the most complicated molecule. We arbitrarily start with the elements in . The silicon is balanced (one atom on each side), but the fluorine is not. To balance the fluorine, we need a coefficient of before the .
Hydrogen and oxygen are not balanced. Because we have four hydrogen atoms on the left and two on the right, we place a before the :
This balances the hydrogen and the oxygen (two atoms on each side).
Step 4
Check 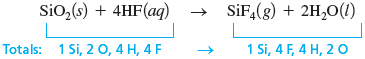
All atoms check, so the equation is balanced.
Self-Check: Exercise 6.3
· Give the balanced equation for each of the following reactions.
a. When solid ammonium nitrite is heated, it produces nitrogen gas and water vapor.
b. Gaseous nitrogen monoxide (common name, nitric oxide) decomposes to produce dinitrogen monoxide gas (common name, nitrous oxide) and nitrogen dioxide gas.
c. Liquid nitric acid decomposes to reddish-brown nitrogen dioxide gas, liquid water, and oxygen gas. (This is why bottles of nitric acid become yellow upon standing.)
See Problems 6.37, 6.38, 6.39, 6.40, 6.41, 6.42, 6.43, and 6.44.