Introductory Chemistry: A Foundation - Zumdahl S.S., DeCoste D.J. 2019
Chemical Reactions: An Introduction
Chapter Review
Key Terms
· chemical reaction (6.2)
· chemical equation (6.2)
· reactants (6.2)
· products (6.2)
· balancing the chemical equation (6.2)
· coefficients (6.3)
For Review
· A chemical reaction produces a signal that it has occurred. These signals include
o Color change
o Solid formation
o Bubble formation
o Heat
o Flame
· The physical states of reactants and products in a reaction are indicated by the following symbols.
|
Physical States |
|
Symbol |
State |
solid |
|
liquid |
|
gas |
|
dissolved in water (in aqueous solution) |
|
· Chemical reactions involve a rearrangement of the ways atoms are grouped together.
· A chemical equation represents a chemical reaction.
o Reactants are shown to the left of an arrow.
o Products are shown to the right of the arrow.
· In a chemical reaction, atoms are neither created nor destroyed. A balanced chemical equation must have the same number of each type of atom on the reactant and product sides.
· A balanced chemical equation uses numbers (coefficients) in front of the reactant and product formulas to show the relative numbers of each.
· A chemical reaction is balanced by using a systematic approach.
o Write the formulas of the reactants and products to give the unbalanced chemical equation.
o Balance by trial and error, starting with the most complicated molecule(s).
o Check to be sure the equation is balanced (same numbers of all types of atoms on the reactant and product sides).
Active Learning Questions
These questions are designed to be considered by groups of students in class. Often these questions work well for introducing a particular topic in class.
· 1.
The following are actual student responses to the question: Why is it necessary to balance chemical equations?
a. The chemicals will not react until you have added the correct ratios.
b. The correct products will not form unless the right amounts of reactants have been added.
c. A certain number of products cannot form without a certain number of reactants.
d. The balanced equation tells you how much reactant you need, and allows you to predict how much product you will make.
e. A ratio must be established for the reaction to occur as written.
Justify the best choice, and, for choices you did not pick, explain what is wrong with them.
· 2.
What information do we get from a formula? From an equation?
· 3.
Given the equation for the reaction: , draw a molecular diagram that represents the reaction (make sure it is balanced).
· 4.
What do the subscripts in a chemical formula represent? What do the coefficients in a balanced chemical equation represent?
· 5.
Can the subscripts in a chemical formula be fractions? Explain.
· 6.
Can the coefficients in a balanced chemical equation be fractions? Explain.
· 7.
Changing the subscripts of chemicals can mathematically balance the equations. Why is this unacceptable?
· 8.
Table 6.1 lists some clues that a chemical reaction has occurred. However, these events do not necessarily prove the existence of a chemical change. Give an example for each of the clues that is not a chemical reaction but a physical change.
· 9.
Use molecular-level drawings to show the difference between physical and chemical changes.
· 10.
It is stated in Section 6.3 of the text that to balance equations by inspection you start “with the most complicated molecule.” What does this mean? Why is it best to do this?
· 11.
Which of the following statements concerning balanced chemical equations are true? There may be more than one true statement.
a. Atoms are neither created nor destroyed.
b. The coefficients indicate the mass ratios of the substances used.
c. The sum of the coefficients on the reactant side always equals the sum of the coefficients on the product side.
· 12.
Consider the generic chemical equation (where a, b, c, and d represent coefficients for the chemicals A, B, C, and D, respectively).
a. How many possible values are there for “c”? Explain your answer.
b. How many possible values are there for “c/d”? Explain your answer.
· 13.
How is the balancing of chemical equations related to the law of conservation of mass?
· 14.
Which of the following correctly describes the balanced chemical equation given below? There may be more than one true statement. If a statement is incorrect, explain why it is incorrect.
a. For every atoms of aluminum that reacts with atoms of oxygen, molecules of aluminum oxide are produced.
b. For every moles of aluminum that reacts with moles of oxygen, moles of aluminum(III) oxide are produced.
c. For every g of aluminum that reacts with g of oxygen, g of aluminum oxide are produced.
· 15.
Which of the following correctly balances the chemical equation given below? There may be more than one correct balanced equation. If a balanced equation is incorrect, explain why it is incorrect.
a.
b.
c.
d.
· 16.
The reaction of an element with element is represented in the following diagram. Which of the elements best describes this reaction?
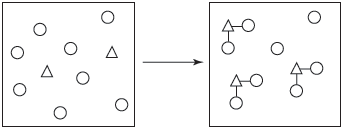
a.
b.
c.
d.
Questions and Problems: 6.1 Evidence for a Chemical Reaction
Questions and Problems with answers below also have full solutions in the Student Solutions Guide.
Questions
· 1.
How do we know when a chemical reaction is taking place? Can you think of an example of how each of the five senses (sight, hearing, taste, touch, smell) might be used in detecting when a chemical reaction has taken place?
· 2.
These days many products are available to whiten teeth at home. Many of these products contain a peroxide that bleaches stains from the teeth. What evidence is there that the bleaching process is a chemical reaction?
· 3.
Although these days many people have “self-cleaning” ovens, if your oven gets really dirty you may have to resort to one of the spray-on oven cleaner preparations sold in supermarkets. What evidence is there that such oven cleaners work by a chemical reaction?
· 4.
Although this is no longer generally recommended, in the past, small cuts and abrasions on the skin were frequently cleaned using hydrogen peroxide solution. What evidence is there that treating a wound with hydrogen peroxide causes a chemical reaction to take place?
· 5.
You have probably had the unpleasant experience of discovering that a flashlight battery has gotten old and begun to leak. Is there evidence that this change is due to a chemical reaction?
· 6.
If you’ve ever left bread in a toaster too long, you know that the bread eventually burns and turns black. What evidence is there that this represents a chemical process?
Questions and Problems: 6.2 Chemical Equations
Questions and Problems with answers below also have full solutions in the Student Solutions Guide.
Questions
· 7.
What are the substances to the left of the arrow in a chemical equation called? To the right of the arrow? What does the arrow itself mean?
· 8.
For the unbalanced chemical equation
a. list the reactant(s).
b. list the product(s).
· 9.
In a chemical reaction, the total number of atoms present after the reaction is complete is (larger than/smaller than/the same as) the total number of atoms present before the reaction began.
· 10.
What does “balancing” an equation accomplish?
· 11.
Why are the physical states of the reactants and products often indicated when writing a chemical equation?
· 12.
The notation “ ” after a substance’s formula indicates it exists in the state.
Problems
Note: In some of the following problems you will need to write a chemical formula from the name of the compound. Review Chapter 5 if you are having trouble.
· 13.
A common experiment to determine the relative reactivity of metallic elements is to place a pure sample of one metal into an aqueous solution of a compound of another metallic element. If the pure metal you are adding is more reactive than the metallic element in the compound, then the pure metal will replace the metallic element in the compound. For example, if you place a piece of pure zinc metal into a solution of copper(II) sulfate, the zinc will slowly dissolve to produce zinc sulfate solution, and the copper(II) ion of the copper(II) sulfate will be converted to metallic copper. Write the unbalanced equation for this process.
· 14.
A common lecture demonstration called “elephant’s toothpaste” demonstrates the reaction of hydrogen peroxide producing water and oxygen gas. Write the unbalanced chemical equation for this process.
· 15.
If a sample of pure hydrogen gas is ignited very carefully, the hydrogen burns gently, combining with the oxygen gas of the air to form water vapor. Write the unbalanced chemical equation for this reaction.
· 16.
Liquid hydrazine, , has been used as a fuel for rockets. When the rocket is to be launched, a catalyst causes the liquid hydrazine to decompose quickly into elemental nitrogen and hydrogen gases. The rapid expansion of the product gases and the heat released by the reaction provide the thrust for the rocket. Write the unbalanced equation for the reaction of hydrazine to produce nitrogen and hydrogen gases.
· 17.
If electricity of sufficient voltage is passed into a solution of potassium iodide in water, a reaction takes place in which elemental hydrogen gas and elemental iodine are produced, leaving a solution of potassium hydroxide. Write the unbalanced equation for this process.
· 18.
Silver oxide may be decomposed by strong heating into silver metal and oxygen gas. Write the unbalanced chemical equation for this process.
· 19.
Elemental boron is produced in one industrial process by heating diboron trioxide with magnesium metal, also producing magnesium oxide as a by-product. Write the unbalanced chemical equation for this process.
· 20.
Many over-the-counter antacid tablets are now formulated using calcium carbonate as the active ingredient, which enables such tablets to also be used as dietary calcium supplements. As an antacid for gastric hyperacidity, calcium carbonate reacts by combining with hydrochloric acid found in the stomach, producing a solution of calcium chloride, converting the stomach acid to water, and releasing carbon dioxide gas (which the person suffering from stomach problems may feel as a “burp”). Write the unbalanced chemical equation for this process.
· 21.
Phosphorus trichloride is used in the manufacture of certain pesticides and may be synthesized by direct combination of its constituent elements. Write the unbalanced chemical equation for this process.
· 22.
Pure silicon, which is needed in the manufacturing of electronic components, may be prepared by heating silicon dioxide (sand) with carbon at high temperatures, releasing carbon monoxide gas. Write the unbalanced chemical equation for this process.
· 23.
Nitrous oxide gas (systematic name: dinitrogen monoxide) is used by some dental practitioners as an anesthetic. Nitrous oxide (and water vapor as by-product) can be produced in small quantities in the laboratory by careful heating of ammonium nitrate. Write the unbalanced chemical equation for this reaction.
· 24.
Solid zinc is added to an aqueous solution containing dissolved hydrogen chloride to produce gaseous hydrogen that bubbles out of the solution and zinc chloride that remains dissolved in the water. Write the unbalanced chemical equation for this process.
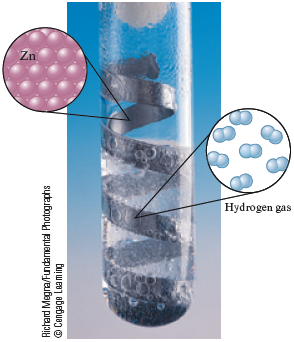
Richard Megna/Fundamental Photographs © Cengage Learning
· 25.
Acetylene gas is often used by plumbers, welders, and glass blowers because it burns in oxygen with an intensely hot flame. The products of the combustion of acetylene are carbon dioxide and water vapor. Write the unbalanced chemical equation for this process.
· 26.
The burning of high-sulfur fuels has been shown to cause the phenomenon of “acid rain.” When a high-sulfur fuel is burned, the sulfur is converted to sulfur dioxide and sulfur trioxide . When sulfur dioxide and sulfur trioxide gas dissolve in water in the atmosphere, sulfurous acid and sulfuric acid are produced, respectively. Write the unbalanced chemical equations for the reactions of sulfur dioxide and sulfur trioxide with water.
· 27.
The Group 2 metals ( , , ) can be produced in the elemental state by the reaction of their oxides with aluminum metal at high temperatures, also producing solid aluminum oxide as a by-product. Write the unbalanced chemical equations for the reactions of barium oxide, calcium oxide, and strontium oxide with aluminum.
· 28.
There are fears that the protective ozone layer around the earth is being depleted. Ozone, , is produced by the interaction of ordinary oxygen gas in the atmosphere with ultraviolet light and lightning discharges. The oxides of nitrogen (which are common in automobile exhaust gases), in particular, are known to decompose ozone. For example, gaseous nitric oxide reacts with ozone gas to produce nitrogen dioxide gas and oxygen gas. Write the unbalanced chemical equation for this process.
· 29.
Carbon tetrachloride was widely used for many years as a solvent until its harmful properties became well established. Carbon tetrachloride may be prepared by the reaction of natural gas (methane, ) and elemental chlorine gas in the presence of ultraviolet light. Write the unbalanced chemical equation for this process.
· 30.
When elemental phosphorus, , burns in oxygen gas, it produces an intensely bright light, a great deal of heat, and massive clouds of white solid phosphorus(V) oxide product. Given these properties, it is not surprising that phosphorus has been used to manufacture incendiary bombs for warfare. Write the unbalanced equation for the reaction of phosphorus with oxygen gas to produce phosphorus(V) oxide.
· 31.
Calcium oxide is sometimes very challenging to store in the chemistry laboratory. This compound reacts with moisture in the air and is converted to calcium hydroxide. If a bottle of calcium oxide is left on the shelf too long, it gradually absorbs moisture from the humidity in the laboratory. Eventually the bottle cracks and spills the calcium hydroxide that has been produced. Write the unbalanced chemical equation for this process.
· 32.
Although they were formerly called the inert gases, the heavier elements of Group 8 do form relatively stable compounds. For example, at high temperatures in the presence of an appropriate catalyst, xenon gas will combine directly with fluorine gas to produce solid xenon tetrafluoride. Write the unbalanced chemical equation for this process.
· 33.
The element tin often occurs in nature as the oxide, . To produce pure tin metal from this sort of tin ore, the ore usually is heated with coal (carbon). This produces pure molten tin, with the carbon being removed from the reaction system as the gaseous byproduct carbon monoxide. Write the unbalanced equation for this process.
· 34.
Nitric acid, , can be produced by reacting high-pressure ammonia gas with oxygen gas at around in the presence of a platinum catalyst. Water is a by-product of the reaction. Write the unbalanced chemical equation for this process.
Questions and Problems: 6.3 Balancing Chemical Equations
Questions and Problems with answers below also have full solutions in the Student Solutions Guide.
Questions
![]() directs you to the Chemistry in Focus feature in the chapter
directs you to the Chemistry in Focus feature in the chapter
· 35.
When balancing chemical equations, beginning students are often tempted to change the numbers within a formula (the subscripts) to balance the equation. Why is this never permitted? What effect does changing a subscript have?
· 36.
![]() The “Chemistry in Focus” segment The Beetle That Shoots Straight discusses the bombardier beetle and the chemical reaction of the decomposition of hydrogen peroxide.
The “Chemistry in Focus” segment The Beetle That Shoots Straight discusses the bombardier beetle and the chemical reaction of the decomposition of hydrogen peroxide.
The balanced equation given in the segment is
Why can’t we balance the equation in the following way?
Use molecular-level pictures like those in Section 6.3 to support your answer.
Problems
· 37.
Balance each of the following chemical equations.
a.
b.
c.
d.
e.
f.
g.
h.
· 38.
Balance the equation for the reaction of potassium with water.

Richard Megna/Fundamental Photographs © Cengage Learning
· 39.
Balance each of the following chemical equations.
a.
b.
c.
d.
e.
f.
g.
h.
· 40.
Balance each of the following chemical equations.
a.
b.
c.
d.
e.
f.
g.
h.
· 41.
Balance each of the following chemical equations.
a.
b.
c.
d.
e.
f.
g.
h.
· 42.
Balance each of the following chemical equations.
a.
b.
c.
d.
e.
f.
g.
h.
· 43.
Balance each of the following chemical equations.
a.
b.
c.
d.
e.
f.
g.
h.
· 44.
Balance each of the following chemical equations.
a.
b.
c.
d.
e.
f.
g.
h.
Additional Problems
· 45.
Acetylene gas, , is used in welding because it generates an extremely hot flame when it is combusted with oxygen. The heat generated is sufficient to melt the metals being welded together. Carbon dioxide gas and water vapor are the chemical products of this reaction. Write the unbalanced chemical equation for the reaction of acetylene with oxygen.
· 46.
When balancing a chemical equation, which of the following statements is false?
a. Subscripts in the reactants must be conserved in the products.
b. Coefficients are used to balance the atoms on both sides.
c. The law of conservation of matter must be followed.
d. Phases are often shown for each compound but are not critical to balancing an equation.
· 47.
Crude gunpowders often contain a mixture of potassium nitrate and charcoal (carbon). When such a mixture is heated until reaction occurs, a solid residue of potassium carbonate is produced. The explosive force of the gunpowder comes from the fact that two gases are also produced (carbon monoxide and nitrogen), which increase in volume with great force and speed. Write the unbalanced chemical equation for the process.
· 48.
The following demonstration takes place in a two-step process: First, solid calcium carbide reacts with liquid water to produce acetylene gas and aqueous calcium hydroxide. Second, the acetylene gas produced is then ignited with a match, causing the combustion reaction of acetylene with oxygen gas to produce gaseous carbon dioxide and gaseous water. Write the balanced equation for each reaction that is occurring.
· 49.
Methanol (methyl alcohol), , is a very important industrial chemical. Formerly, methanol was prepared by heating wood to high temperatures in the absence of air. The complex compounds present in wood are degraded by this process into a charcoal residue and a volatile portion that is rich in methanol. Today, methanol is instead synthesized from carbon monoxide and elemental hydrogen. Write the balanced chemical equation for this latter process.
· 50.
The Hall process is an important method by which pure aluminum is prepared from its oxide (alumina, ) by indirect reaction with graphite (carbon). Balance the following equation, which is a simplified representation of this process.
· 51.
Iron oxide ores, commonly a mixture of and , are given the general formula . They yield elemental iron when heated to a very high temperature with either carbon monoxide or elemental hydrogen. Balance the following equations for these processes.
· 52.
True or false? Coefficients can be fractions when balancing a chemical equation. Whether true or false, explain why this can or cannot occur.
o True
o False
· 53.
When steel wool (iron) is heated in pure oxygen gas, the steel wool bursts into flame and a fine powder consisting of a mixture of iron oxides ( and ) forms. Write separate unbalanced equations for the reaction of iron with oxygen to give each of these products.
· 54.
One method of producing hydrogen peroxide is to add barium peroxide to water. A precipitate of barium oxide forms, which may then be filtered off to leave a solution of hydrogen peroxide. Write the balanced chemical equation for this process.
· 55.
When elemental boron, , is burned in oxygen gas, the product is diboron trioxide. If the diboron trioxide is then reacted with a measured quantity of water, it reacts with the water to form what is commonly known as boric acid, . Write a balanced chemical equation for each of these processes.
· 56.
A common experiment in introductory chemistry courses involves heating a weighed mixture of potassium chlorate, , and potassium chloride. Potassium chlorate decomposes when heated, producing potassium chloride and evolving oxygen gas. By measuring the volume of oxygen gas produced in this experiment, students can calculate the relative percentage of and in the original mixture. Write the balanced chemical equation for this process.
· 57.
A common demonstration in chemistry courses involves adding a tiny speck of manganese(IV) oxide to a concentrated hydrogen peroxide, , solution. Hydrogen peroxide is unstable, and it decomposes quite spectacularly under these conditions to produce oxygen gas and steam (water vapor). Manganese(IV) oxide is a catalyst for the decomposition of hydrogen peroxide and is not consumed in the reaction. Write the balanced equation for the decomposition reaction of hydrogen peroxide.
· 58.
Write a balanced chemical equation for the complete combustion of pentene, . In combustion, pentene reacts with oxygen to produce carbon dioxide and water.
· 59.
Glass is a mixture of several compounds, but a major constituent of most glass is calcium silicate, . Glass can be etched by treatment with hydrogen fluoride: attacks the calcium silicate of the glass, producing gaseous and water-soluble products (which can be removed by washing the glass). Balance the following equation for the reaction of hydrogen fluoride with calcium silicate.

Richard Megna/Fundamental Photographs © Cengage Learning
· 60.
Balance the following chemical equation.
· 61.
If you had a “sour stomach,” you might try an over-the-counter antacid tablet to relieve the problem. Can you think of evidence that the action of such an antacid is a chemical reaction?
· 62.
When iron wire is heated in the presence of sulfur, the iron soon begins to glow, and a chunky, blue-black mass of iron(II) sulfide is formed. Write the unbalanced chemical equation for this reaction.
· 63.
When finely divided solid sodium is dropped into a flask containing chlorine gas, an explosion occurs and a fine powder of sodium chloride is deposited on the walls of the flask. Write the unbalanced chemical equation for this process.
· 64.
If aqueous solutions of potassium chromate and barium chloride are mixed, a bright yellow solid (barium chromate) forms and settles out of the mixture, leaving potassium chloride in solution. Write a balanced chemical equation for this process.
· 65.
When hydrogen sulfide, , gas is bubbled through a solution of lead(II) nitrate, , a black precipitate of lead(II) sulfide, , forms, and nitric acid, , is produced. Write the unbalanced chemical equation for this reaction.
· 66.
If an electric current is passed through aqueous solutions of sodium chloride, sodium bromide, and sodium iodide, the elemental halogens are produced at one electrode in each case, with hydrogen gas being evolved at the other electrode. If the liquid is then evaporated from the mixture, a residue of sodium hydroxide remains. Write balanced chemical equations for these electrolysis reactions.
· 67.
When a strip of magnesium metal is heated in oxygen, it bursts into an intensely white flame and produces a finely powdered dust of magnesium oxide. Write the unbalanced chemical equation for this process.
· 68.
Which of the following statements is false for the reaction of hydrogen gas with oxygen gas to produce water? (a, b, and c represent coefficients)
a. The ratio of “ ” must always equal one.
b. The sum of equals when balanced using the lowest whole-number coefficients.
c. Coefficient b can equal because coefficients can be fractions.
d. The number of atoms on the reactant side must equal the number of atoms on the product side.
e. Subscripts can be changed to balance this equation, just as they can be changed to balance the charges when writing the formula for an ionic compound.
· 69.
When solid red phosphorus, , is burned in air, the phosphorus combines with oxygen, producing a choking cloud of tetraphosphorus decoxide. Write the unbalanced chemical equation for this reaction.
· 70.
When copper(II) oxide is boiled in an aqueous solution of sulfuric acid, a strikingly blue solution of copper(II) sulfate forms along with additional water. Write the unbalanced chemical equation for this reaction.
· 71.
When lead(II) sulfide is heated to high temperatures in a stream of pure oxygen gas, solid lead(II) oxide forms with the release of gaseous sulfur dioxide. Write the unbalanced chemical equation for this reaction.
· 72.
Which of the following statements about chemical reactions is false?
a. When balancing a chemical equation, all subscripts must be conserved.
b. When one coefficient is doubled, the rest of the coefficients in the balanced equation must also be doubled.
c. The subscripts in a balanced equation tell us the number of atoms in a molecule.
d. The phases in a chemical reaction tell us the nature of the reactants and products.
· 73.
Balance each of the following chemical equations.
a.
b.
c.
d.
e.
f.
g.
h.
· 74.
Balance the following chemical equation:
· 75.
Balance each of the following chemical equations.
a.
b.
c.
d.
e.
f.
g.
h.
· 76.
Using different shapes to distinguish between different elements, draw a balanced equation for the following reaction at the microscopic level.
ChemWork Problems
These multiconcept problems (and additional ones) are found interactively online with the same type of assistance a student would get from an instructor.
· 77.
Which of the following statements about chemical equations is (are) true?
a. When balancing a chemical equation, you can never change the coefficient in front of any chemical formula.
b. The coefficients in a balanced chemical equation refer to the number of grams of reactants and products.
c. In a chemical equation, the reactants are on the right, and the products are on the left.
d. When balancing a chemical equation, you can never change the subscripts of any chemical formula.
e. In chemical reactions, matter is neither created nor destroyed, so a chemical equation must have the same number of atoms on both sides of the equation.
· 78.
Balance the following chemical equations.
· 79.
Balance the following chemical equations.